Amphiphilic self-assembled nanomicelle based on nano Low-generation PAMAM (polyamidoamine) dendrimer and application thereof
A nanometer self-assembly, dendrimer technology, which is applied in the directions of non-active ingredients medical preparations, active ingredients-containing medical preparations, and drug combinations, etc. problem, to achieve the effect of improving poor solubility, simple synthesis steps and easy hemolysis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
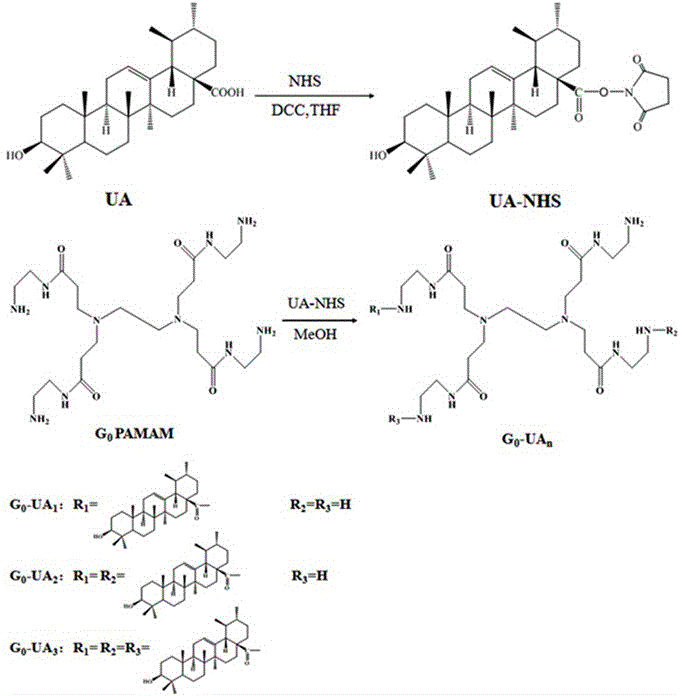
[0072] Example 1 Synthesis of ursolic acid-modified amphiphilic dendrimer conjugates
[0073] (1) Based on G 0 Synthesis of amphiphilic conjugates of PAMAM dendrimers (G 0 -UA 2 example)
[0074] ① Synthesis of UA-NHS
[0075]Take 2.28g (5mmol) ursolic acid (abbreviation: UA, the same below) in a 150mL round bottom flask, add 50mL tetrahydrofuran, after the UA is dissolved, add 1.15g (10mmol) NHS dissolved in 10mL acetonitrile, and then 2.06 g (10 mmol) of DCC dissolved in 25 mL of tetrahydrofuran was added dropwise at 15°C. Stir magnetically at room temperature for 24 h. After the reaction was completed, the precipitate was removed by filtration, and then tetrahydrofuran was removed by a rotary evaporator to obtain a white powder crude product. Purified UA-NHS was obtained by silica gel column chromatography, and the eluent was dichloromethane:methanol (v / v)=50:1. Its high-resolution mass spectrum see figure 1 .
[0076] ②G O -UA 2 Synthesis
[0077] Take 0.44g (...
Embodiment 2
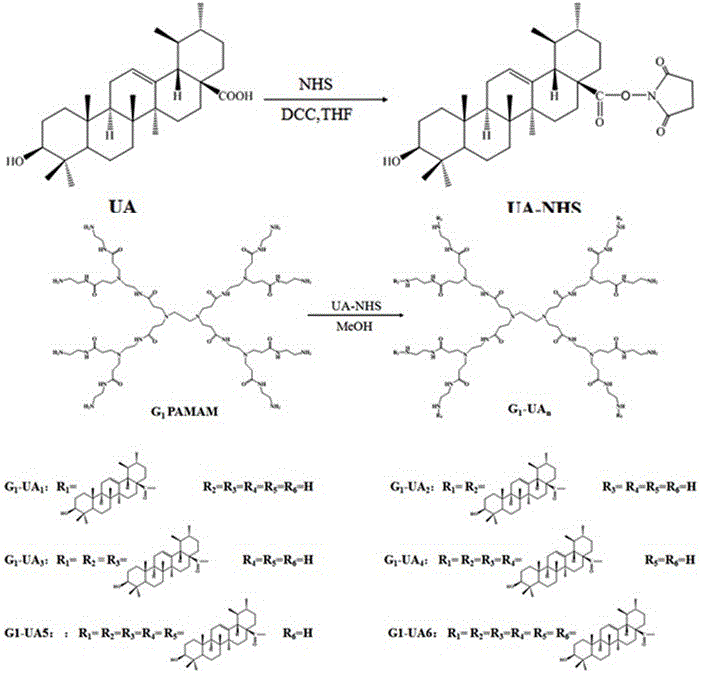
[0083] Example 2 Synthesis of methotrexate-modified amphiphilic dendrimer conjugates (based on G 1 PAMAM Dendrimer, G 1 -Me 1 example)
[0084] Take 0.182g (0.4mmol) of methotrexate (Methotrexate abbreviation: Me, the same below) in a 150mL round bottom flask, add 50mL of N,N-dimethylformamide to dissolve, add 0.115g dissolved in 10mL of acetonitrile ( 10mmol) NHS, and then dropwise added 0.206g (10mmol) DCC dissolved in 25mL THF at 10~15°C. Stir magnetically at room temperature for 24 h. After the reaction, the precipitate was removed by filtration to obtain an organic solution of the active intermediate product; 1 PAMAM solution in ice bath. At room temperature, avoid light and magnetically stir for 48h. After the reaction, the reaction solution was dialyzed in N,N-dimethylformamide (MWCO=1500) for 1~3 days, then dialyzed in water for 3 days, and freeze-dried to obtain methotrexate-modified amphiphilic dendrimers. molecular conjugates.
Embodiment 3
[0085] Example 3 Synthesis of aspirin-modified amphiphilic dendrimer conjugates (based on G 0 PAMAM Dendrimer, G 0 -ASP 1 example)
[0086] Take 72mg (0.4mmol) of aspirin (Aspirin abbreviation: ASP, the same below) in a 150mL round bottom flask, add 50mL of dichloromethane to dissolve, add 0.115g (10mmol) of NHS dissolved in 10mL of acetonitrile, and then 0.206 g (10 mmol) of DCC dissolved in 25 mL of tetrahydrofuran was added dropwise. Stir magnetically at room temperature for 24 h. After the reaction, the precipitate was removed by filtration to obtain an organic solution of the active intermediate product; 0 PAMAM solution in ice bath. At room temperature, avoid light and magnetically stir for 48h. After the reaction, the reaction solution was dialyzed in N,N-dimethylformamide (MWCO=500) for 1~3 days, then dialyzed in water for 3 days, and freeze-dried to obtain the methotrexate-modified amphiphilic dendrimers. molecular conjugates.
PUM
| Property | Measurement | Unit |
|---|---|---|
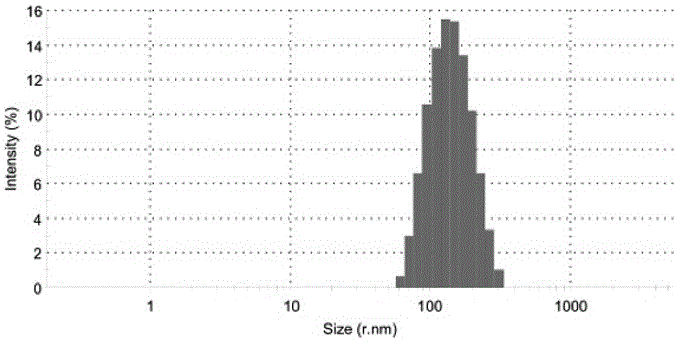
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com