Oral medicinal composition of glycyrrhizic acid or glycyrrhetate and preparation method thereof
A technology of composition and glycyrrhizic acid, which is applied in the direction of drug combination, medical formula, medical preparations containing active ingredients, etc., can solve the problems of affecting the full contact of drugs and affecting drug absorption, so as to improve bioavailability, improve curative effect, The effect of effective absorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
[0046] Preparation Example 1: 18-α magnesium glycyrrhizinate (magnesium isoglycyrrhizinate) enteric-coated pellets and capsules
[0047] Pellet Prescription:
[0048] Component name 1000 capsule dosage (g)
[0049] Magnesium 18-α Glycyrrhizinate 50 (as C 42 h 60 MgO 16 count)
[0050] Medicinal blank pellet core (sucrose type) 100
[0051] Soy Lecithin 50
[0052] Polyethylene glycol 6000 0.16
[0053] Talc 5
[0054] Proper amount of ethanol
[0055] Prescription of enteric coating solution:
[0056] Component name Amount per 1000 grams of pellets (g)
[0057] Opadry (ACRYC-EZE MP) 300
[0058] water 2000
[0059] Production Process:
[0060] 1. After dissolving the prescribed amount of 18-α magnesium glycyrrhizinate and soybean lecithin in ethanol (if necessary, a small amount of water can be added dropwise to dissolve), evaporate the ethanol to dryness to obtain a compound of 18-α magnesium glycyrrhizinate and soybean lecithin;
[0061] 2. Disperse the compoun...
preparation Embodiment 2
[0066] Preparation Example 2: Diammonium 18-α Glycyrrhizinate Enteric-Coated Pellet Capsules
[0067] Pellet Prescription:
[0068] Component name 1000 capsule dosage (g)
[0069] 18-α diammonium glycyrrhizinate 50(C 42 h 68 N 2 o 16 count)
[0070] Polyene Phosphatidylcholine 25
[0071] Microcrystalline Cellulose 100
[0072] Low-substituted hydroxypropyl cellulose 100
[0073] Talc 37.5
[0074] Proper amount of ethanol
[0075] 15% alcohol solution in proper amount
[0076] Prescription of enteric coating solution:
[0077] Component name Amount per 1000 grams of pellets (g)
[0078] Opadry (ACRYC-EZE MP) 300
[0079] water 2000
[0080] Production Process:
[0081] 1. Dissolve the prescription amount of 18-α diammonium glycyrrhizinate and polyene phosphatidylcholine in ethanol (if necessary, a small amount of water can be added dropwise to dissolve) and evaporate the ethanol to dryness to obtain 18-α diammonium glycyrrhizinate and polyene phosphatidylcholin...
preparation Embodiment 3
[0088] Preparation Example 3: Dipotassium 18-β Glycyrrhizinate Enteric-Coated Pellet Capsules
[0089] Pellet Prescription:
[0090] Component name 1000 capsule dosage (g)
[0091] Dipotassium 18-β Glycyrrhizinate 100(C 42 h 60 K 2 o 16 count)
[0092] Phosphatidylcholine 30
[0093] Lactose 30
[0094] Microcrystalline Cellulose 100
[0095] Low-substituted hydroxypropyl cellulose 5
[0097] Proper amount of ethanol
[0098] 30% alcohol solution in proper amount
[0099] Prescription of enteric coating solution:
[0100] Component name Amount per 1000 grams of pellets (g)
[0101] EUDRAGIT (L100-55) 300
[0102] water 2000
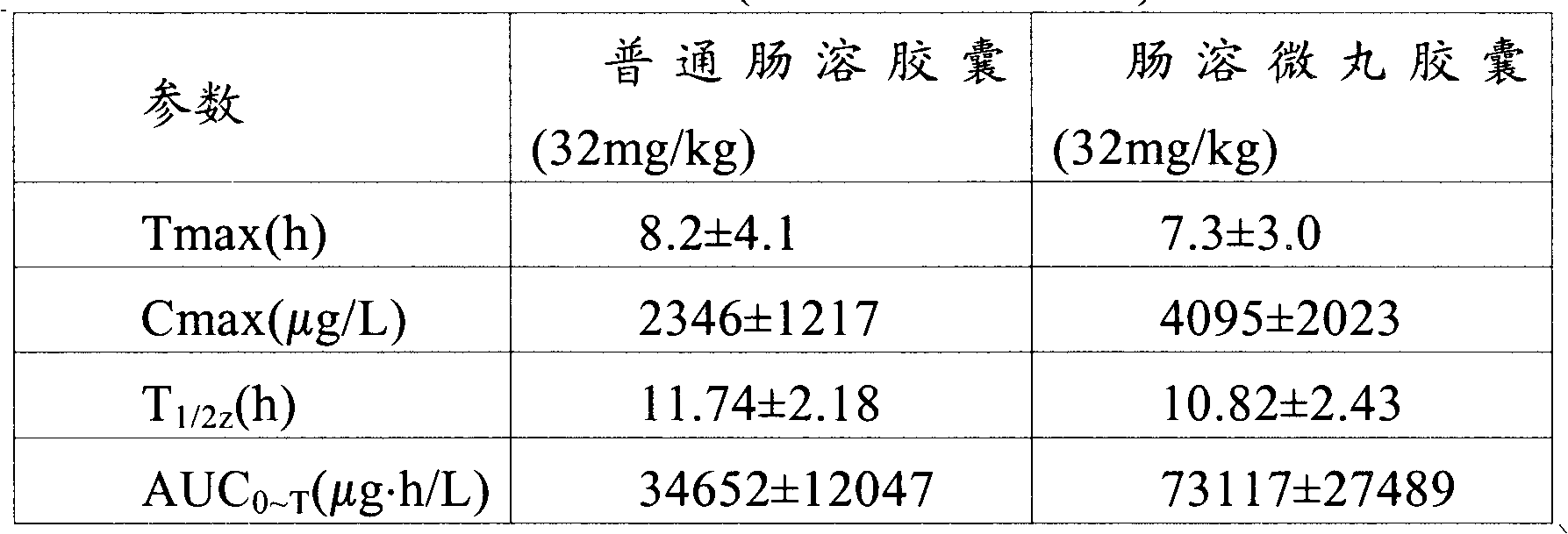
[0103] Using the production process described in Preparation Example 2, using the various components of the prescription amount described in the above prescription to prepare enteric-coated pellets with a diameter of 0.5mm to 1.5mm, and then measuring the drug content of the enteric-coated pellets Filling capsules. ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com