Method for preparing europium-doped calcium titanate CaTiO3: Eu3 plus fluorescent powder by solvent-thermal method
A solvothermal method and fluorescent powder technology, applied in chemical instruments and methods, luminescent materials, etc., can solve the problems of low luminous efficiency and achieve high chemical stability, small size, and good dispersion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
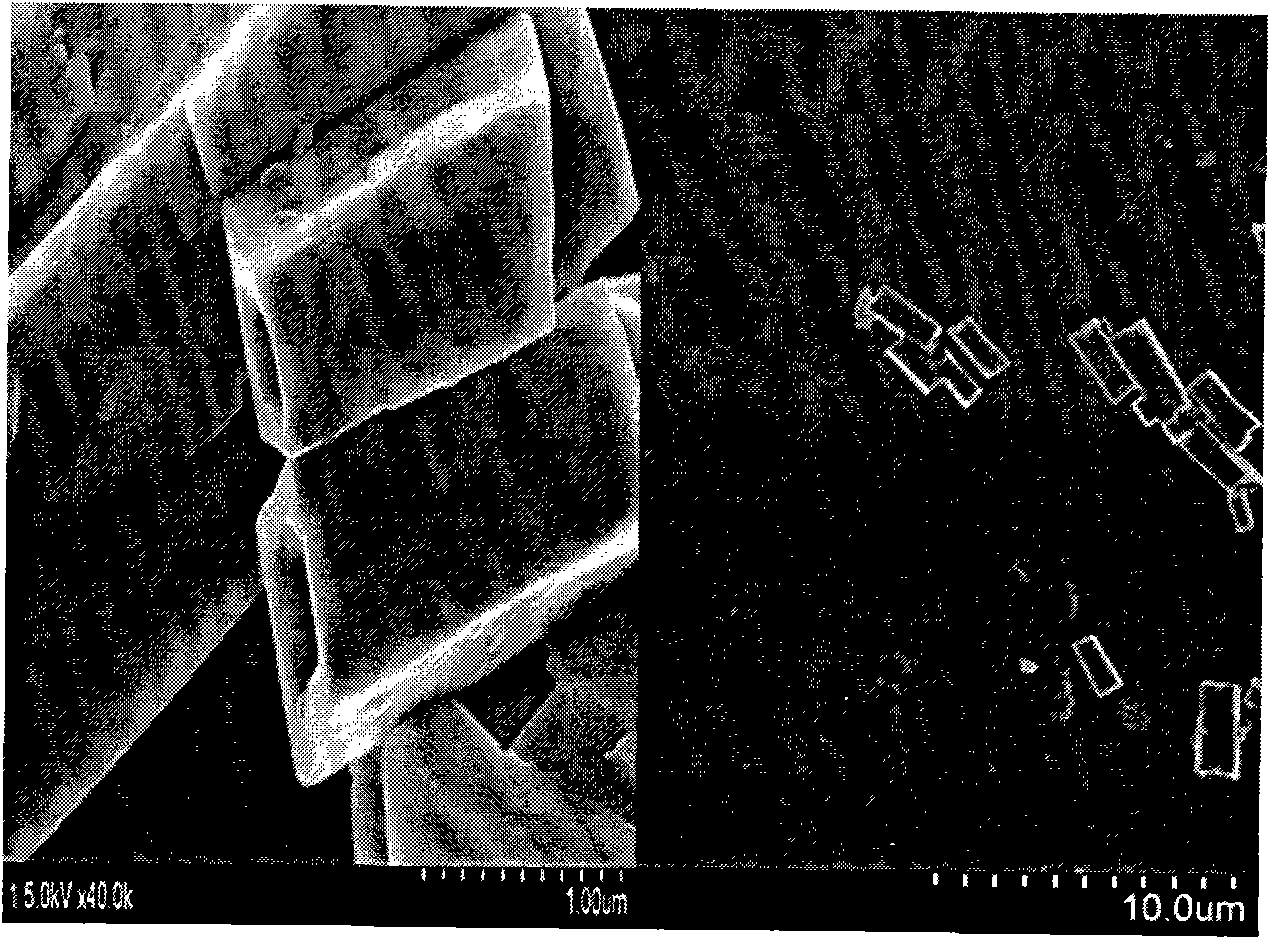
[0021] Measure 1.716ml of n-tetrabutyl titanate, dissolve it in 10ml of absolute ethanol, add 50ml of 0.5mol / L ammonia solution ammonia water dropwise to make it all react as titanium hydroxide precipitate, and wash with water three times to remove excess ammonia ions. Then add 25ml of ethanolamine, 25ml of ethylenediamine, 5ml of deionized water, 2.6163g of calcium acetate, 0.05069g of europium nitrate, 3.3g of sodium hydroxide, and 1ml of polyethylene glycol, and stir for 30min at a rotation speed of 350r / min, so that All solutes are completely dispersed. The above solution and precipitate were poured into a reaction kettle with a volume of 80ml, then put into an oven, kept at 180°C for 14 hours, and then cooled to room temperature. After the reaction, the product was collected by centrifugation, washed with deionized water and hydrochloric acid with pH = 2-3, and then dried at 60°C for 24 hours to obtain CaTiO 3 :Eu 3+ Phosphor.
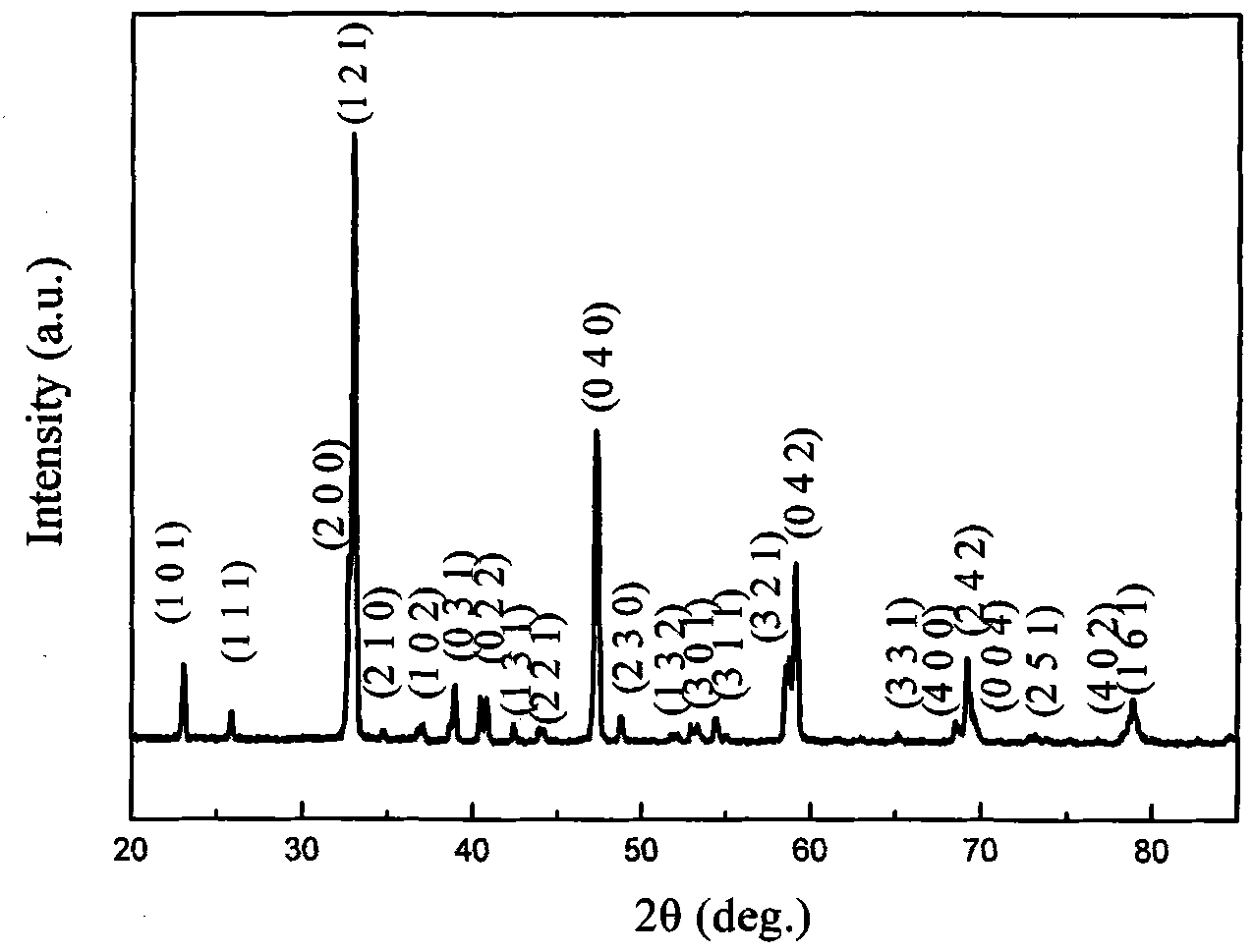
[0022] figure 1 It is the X-ray diffr...
Embodiment 2
[0026] Measure 1.716ml of n-tetrabutyl titanate, dissolve it in 20ml of absolute ethanol, add 25ml of 1mol / L ammonia water dropwise to make it all react into titanium hydroxide precipitation, and wash with water three times to remove excess ammonia ions. Add 20ml of ethanolamine, 20ml of ethylenediamine, 3.5ml of deionized water, 2.5370g of calcium acetate, 0.2028g of europium nitrate, 3.8g of sodium hydroxide, and 1.4ml of polyethylene glycol, and stir for 25min at a rotation speed of 400r / min. , so that all solutes are completely dispersed. The above solution and precipitate were poured into a reaction kettle with a volume of 80ml, then put into an oven, kept at 200°C for 12 hours, and then cooled to room temperature. After the reaction, the product was collected by centrifugation, and the product was washed with deionized water and hydrochloric acid with a pH of 2 to 3, and then dried at 60°C for 28 hours to obtain CaTiO 3 :Eu 3+ Phosphor.
[0027] The X-ray test results...
Embodiment 3
[0031]Measure 1.716ml of n-tetrabutyl titanate, dissolve it in 30ml of absolute ethanol, add 8ml of 3mol / L ammonia water dropwise to make it all react to precipitate titanium hydroxide, and wash with water three times to remove excess ammonia ions. Then add 15ml of ethanolamine, 15ml of ethylenediamine, 3ml of deionized water, 2.3780g of calcium acetate, 0.5069g of europium nitrate, 4.7g of sodium hydroxide, and 1ml of polyethylene glycol, and stir for 20min at a rotation speed of 500r / min, so that All solutes are completely dispersed. The above solution and precipitate were poured into a reaction kettle with a volume of 80ml, then put into an oven, kept at 220°C for 10 hours, and then cooled to room temperature. After the reaction, the product was collected by centrifugation, and the product was washed with deionized water and hydrochloric acid with pH = 2-3, and then dried at 80°C for 20 hours to obtain CaTiO 3 :Eu 3+ Phosphor.
[0032] The X-ray test results show that th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com