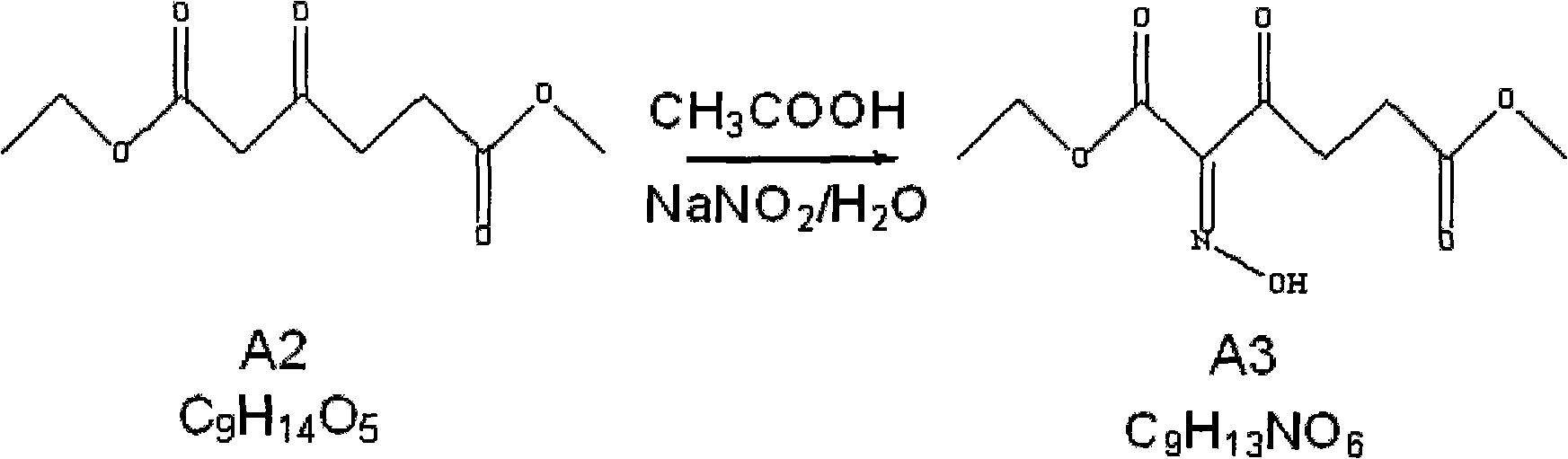
Method for preparing 5-aminolevulinic acid hydrochloride
A technology of aminolevulinic acid hydrochloride and potassium salt, which is applied in the field of medicine, can solve the problems of operator injury, high cost, and low reaction yield, and achieve the effects of industrial production safety, short reaction cycle, and low reaction cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
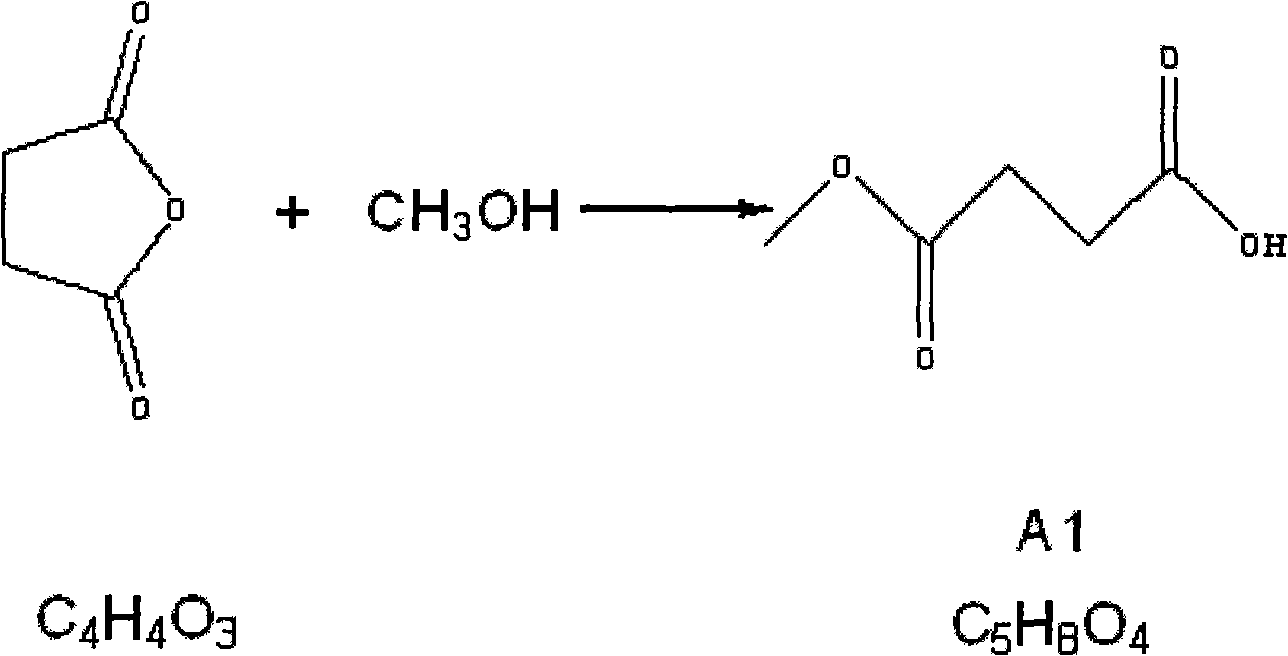
[0029] 1.1 Preparation of compound A1 (monomethyl succinate) (succinic anhydride: methanol = 1: 2.5)
[0030] Add 20Kg (634.2mol) of anhydrous methanol into a 200L reaction kettle, start stirring, add 50Kg (499.65mol) of succinic anhydride, add 20Kg (634.2mol) of anhydrous methanol, turn on the steam and heat up to 75°C for 2 hours, wait The solution was clarified and the steam was turned off, and the methanol was distilled off under normal pressure and then under reduced pressure. Add 10-12Kg of ethyl acetate to the residue, stir evenly, cool (0-5°C), and crystallize overnight. Suction filtration the next day, followed by washing with 1kg of ethyl acetate and 1kg of petroleum ether, and draining to obtain 40Kg of white crystals. Purity (GC detection): >96%, yield: 60.6%.
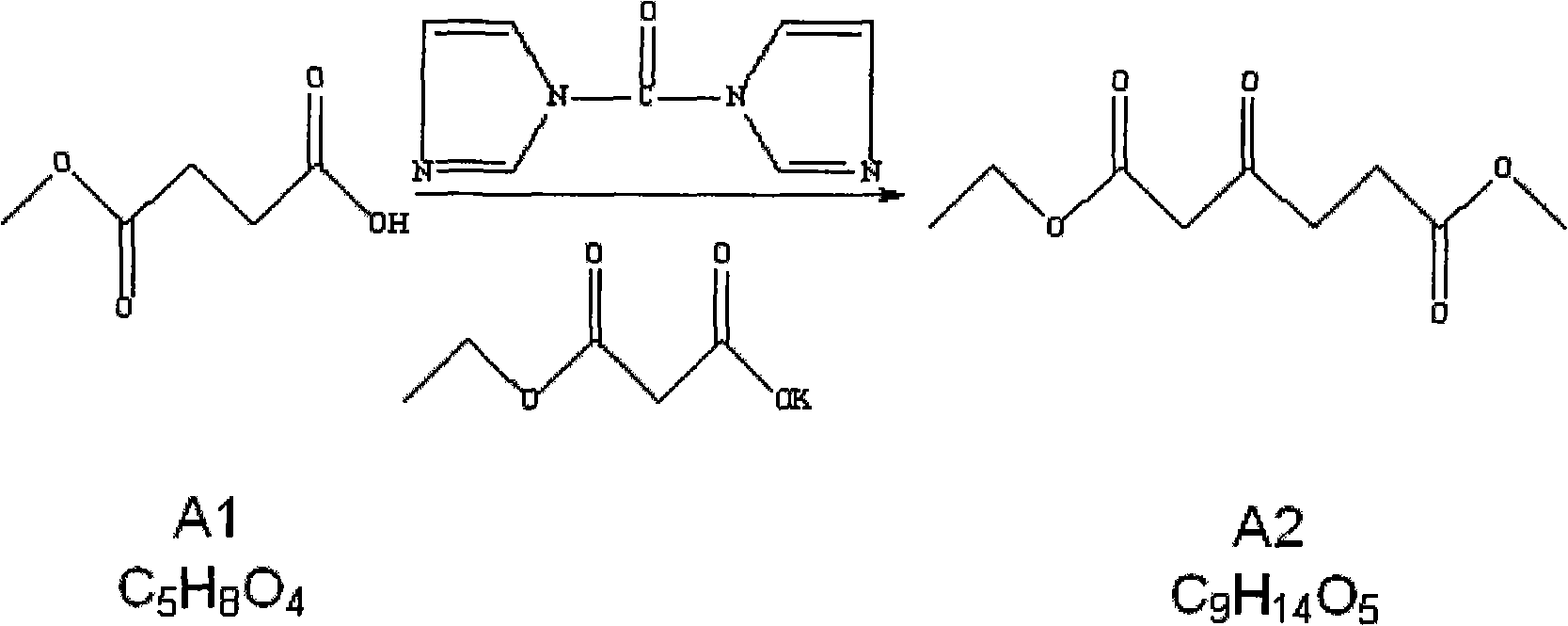
[0031] 1.2 Preparation of compound A2 (1-ethyl 6-methyl 3-oxohexanediate) (compound A1: monoethyl malonate potassium salt = 1: 1.1)
[0032] Add tetrahydrofuran 42Kg (dry) in the reactor of 200L, under s...
Embodiment 2
[0041] 2.1 Preparation of compound A1 (monomethyl succinate) (succinic anhydride:methanol=1:3)
[0042] Add 24Kg (749.5mol) of anhydrous methanol to a 200L reactor, start stirring, add 50Kg (499.65mol) of succinic anhydride, and then add 24Kg (749.5mol) of anhydrous methanol, turn on the steam and heat up to 77°C for 2.5 hours. The solution was clarified and the steam was turned off, and the methanol was distilled off under normal pressure and then under reduced pressure. Add 10-12Kg of ethyl acetate to the residue, stir evenly, cool (0-5°C), and crystallize overnight. Suction filtration the next day, followed by washing with 1kg of ethyl acetate and 1kg of petroleum ether, and draining to obtain 40Kg of white crystals. Purity (GC detection): >96%, yield: 60.6%.
[0043] 2.2 Preparation of compound A2 (1-ethyl 6-methyl 3-oxohexanediate) (compound A1: monoethyl malonate potassium salt = 1:1)
[0044] Add tetrahydrofuran 42Kg (dry) in the reactor of 200L, under stirring situa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com