Veterinary compound sulfadiazine sodium injection and preparation method thereof
A technology of sulfadiazine sodium and injection, which is applied in the field of veterinary compound sulfadiazine sodium injection, can solve the problems of incomplete curative effect and achieve the effect of relieving the surface symptoms of livestock and poultry, high curative effect and accelerated absorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] A compound sulfadiazine sodium injection for veterinary use, the components are as follows:
[0038] Sulfadiazine sodium 10g, enrofloxacin 10g, trimethoprim 4.5g, flunixin meglumine 0.5g, dexamethasone sodium phosphate 0.05g, sodium thiosulfate 0.15g, edetate disodium 0.04 g, benzyl alcohol 20g, N,N-dimethylformamide 50g, water for injection 20g;
[0039] The veterinary compound sulfadiazine sodium injection is prepared by the following steps:
[0040] 1) Dissolve 0.5 g of flunixin meglumine, 0.05 g of dexamethasone sodium phosphate, 0.15 g of sodium thiosulfate, 0.04 g of disodium edetate, and 10 g of sulfadiazine sodium in 20 g of water for injection, and mix solution;
[0041] 2) dissolving 10 g of enrofloxacin in 20 g of benzyl alcohol to obtain a solution of enrofloxacin;
[0042] 3) Dissolving 4.5 g of trimethoprim in 50 g of N,N-dimethylformamide to obtain a trimethoprim solution;
[0043] 4) Mix the mixed solution solution prepared in step 1), the enrofloxac...
Embodiment 2
[0062] A compound sulfadiazine sodium injection for veterinary use, the components are as follows:
[0063] Sulfadiazine sodium 5g, enrofloxacin 15g, trimethoprim 2-8g, flunixin meglumine 0.3g, dexamethasone sodium phosphate 0.02g, sodium thiosulfate 0.1g, edetate disodium 0.03g, 10g benzyl alcohol, 45g N,N-dimethylformamide, 30g water for injection;
[0064] The preparation steps were the same as in Example 1 to obtain compound sulfadiazine sodium injection for veterinary use, with a pH of 9.5.
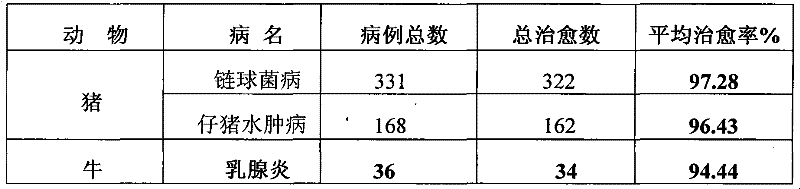
[0065] Table 1 clinical treatment effect of the present invention
[0066]
[0067] Table 2 The present invention and sulfadiazine sodium injection clinical treatment comparative test result
[0068]
[0069]
[0070] The stability test of product is with embodiment 1.
[0071]
[0072] As can be seen from the above data, the product of this embodiment has no obvious change in product color after 3 months of product acceleration test, and is all within the speci...
Embodiment 3
[0074] A compound sulfadiazine sodium injection for veterinary use, the components are as follows:
[0075] Sulfadiazine sodium 7g, enrofloxacin 13g, trimethoprim 4g, flunixin meglumine 0.35g, dexamethasone sodium phosphate 0.05g, sodium thiosulfate 0.15g, edetate disodium 0.03g , benzyl alcohol 15g, N,N-dimethylformamide 45g, water for injection 30g;
[0076] The preparation steps were the same as in Example 1 to obtain compound sulfadiazine sodium injection for veterinary use, with a pH of 9.7.
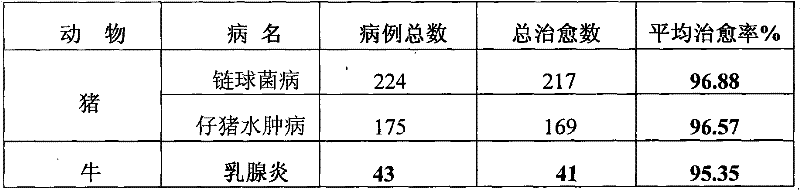
[0077] Table 1 clinical treatment effect of the present invention
[0078]
[0079] Table 2 The present invention and sulfadiazine sodium injection clinical treatment comparative test result
[0080]
[0081]
[0082] The stability test of product is with embodiment 1.
[0083]
[0084] As can be seen from the above data, the product of this embodiment has no obvious change in product color after 3 months of product acceleration test, and is all within the spec...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com