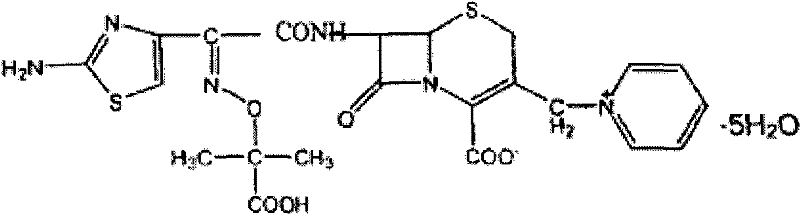
Ceftazidime medicinal composition for injection and preparation method thereof
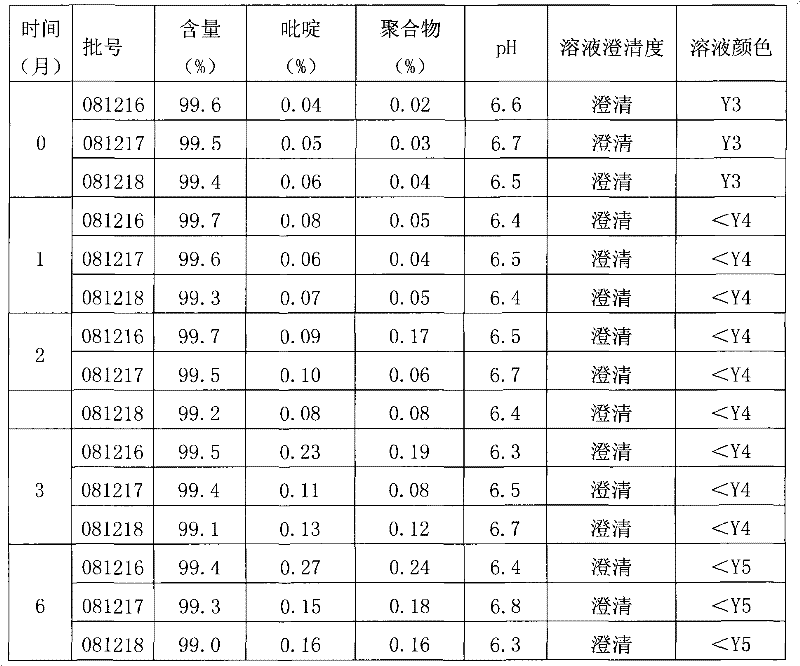
A technology of ceftazidime and its composition, which is applied in the field of ceftazime pharmaceutical composition for injection and its preparation, can solve the problems of ceftazidime color, degradation, polymerization and stability without a good solution, causing great danger to patients, and instability of ceftazidime, etc. , to achieve the effect of solving the problem of high polymer content, high polymer content and stable color
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0078] prescription:
[0079] Ceftazidime 1000g
[0080] Arginine 50g
[0081] L-cysteine 0.1g
[0082] Made 1000 pieces
[0083] Preparation:
[0084] 1) After the raw and auxiliary materials are removed from the outer packaging, they are dust-cleaned, wiped and sterilized, and put into a sterile room for standby;
[0085] 2) In the clean area, the ceftazidime, arginine and L-cysteine are respectively pulverized, sieved, and set aside;
[0086] 3) Take the ceftazidime, arginine and L-cysteine obtained in step 2) in the clean area, mix them evenly according to the ratio of the prescription, pack them in control bottles after the measured content is qualified, press the stopper, roll the cap, After passing the light inspection and inspection, it can be labeled and packaged.
Embodiment 2
[0088] prescription:
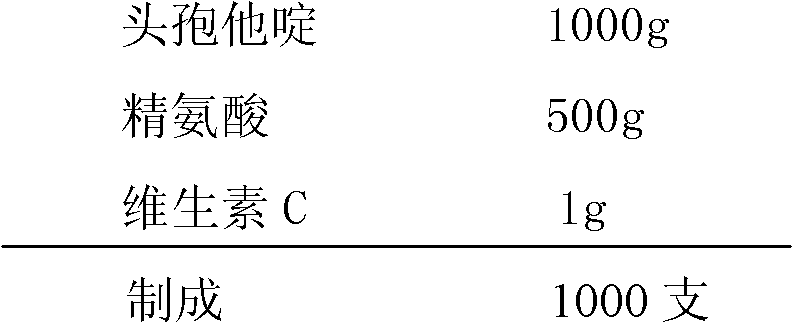
[0089] Ceftazidime 1000g
[0090] Arginine 500g
[0091] Vitamin C 1g
[0092] Made 1000 pieces
[0093] Preparation:
[0094] 1) After the raw and auxiliary materials are removed from the outer packaging, they are dust-cleaned, wiped and sterilized, and put into a sterile room for standby;
[0095] 2) In the clean area, the ceftazidime, arginine and vitamin C are respectively pulverized, sieved, and set aside;
[0096] 3) Weigh the ceftazidime, arginine and vitamin C obtained in step 2) in the clean area, mix them evenly according to the ratio of the prescription, pack them into control bottles after the content is qualified, press the stopper, cap, light check, and test Qualified, label and package.
Embodiment 3
[0098] prescription:
[0099] Ceftazidime 1000g
[0100] Arginine 100g
[0102] Made 1000 pieces
[0103] Preparation:
[0104] 1) After the raw and auxiliary materials are removed from the outer packaging, they are dust-cleaned, wiped and sterilized, and put into a sterile room for standby;
[0105] 2) In the clean area, the ceftazidime, arginine and sodium bisulfite are pulverized and sieved respectively, and set aside;
[0106]3) Weigh the ceftazidime, arginine and sodium bisulfite obtained in step 2) in the clean area, mix them evenly according to the ratio of the prescription, pack them into control bottles after the measured content is qualified, press the stopper, roll the cap, and light check , Pass the inspection, label and pack.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com