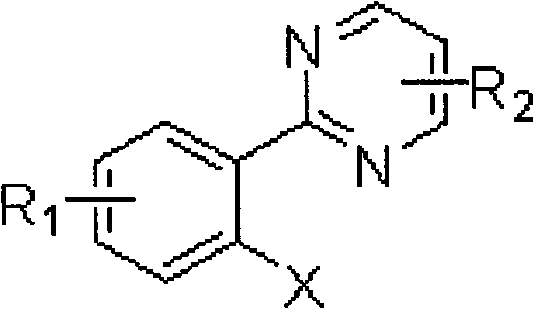
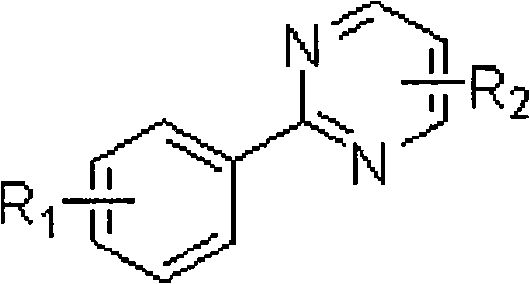
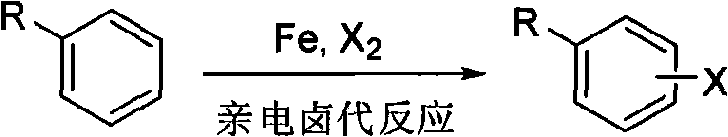Aryl pyrimidine ortho-single halogen substituted compound and synthetic method thereof
A technology for arylpyrimidines and compounds, which is applied in the field of arylpyrimidine ortho-halogen substitution compounds and their synthesis, can solve the problems of harsh reaction conditions, large limitations, and high toxicity of halogenated reagents, and achieve high reaction selectivity, The effect of environmental protection and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Example 1: Preparation of 2-(2-chloro-4-ethoxycarbonylphenyl)pyrimidine
[0047] 2-(2-chloro-4-ethoxycarbonylphenyl) pyrimidine adopts the following steps: 1. add 1 gram of 2-(4-ethoxycarbonylphenyl) pyrimidine, 500 mg of palladium acetate in a 250 ml round bottom flask, 16 grams of additive copper trifluoroacetate, 30 grams of calcium chloride, 100 milliliters of acetic acid, heated to 80 ° C. Track the reaction with thin-layer chromatography until the reaction raw material 2-(4-ethoxycarbonylphenyl)pyrimidine disappears; 2. after the reaction is finished, remove the solvent with a rotary evaporator, add saturated sodium bicarbonate solution to the system, and use acetic acid Extract the product with ethyl ester, and remove the solvent with a rotary evaporator after drying to obtain a crude product; ③The crude product is purified by column chromatography (petroleum ether:ethyl acetate=15:1) to obtain 10.2 grams of 2-(2-chloro-4 -ethoxycarbonylphenyl)pyrimidine in 89% ...
Embodiment 2
[0053] Embodiment two: the method of 2-(2-chloro-4-trifluoromethylphenyl) pyrimidine
[0054] 2-(2-chloro-4-trifluoromethylphenyl) pyrimidine adopts the following steps: 1. add 1 gram of 2-(-4-trifluoromethylphenyl) pyrimidine in a 250 ml round bottom flask, 500 mg Palladium acetate, 15 grams of additive copper trifluoroacetate, 32 grams of calcium chloride, 100 milliliters of acetic acid, heated to 80 ° C, follow the reaction with thin layer chromatography, to the reaction raw material 2-(-4-trifluoromethylphenyl ) pyrimidine disappears; 2. After the reaction is finished, remove the solvent with a rotary evaporator, add saturated sodium bicarbonate solution to the system, extract the product with ethyl acetate, remove the solvent with a rotary evaporator after drying, and obtain a crude product; 3. Use a rotary evaporator for the crude product Purification by column chromatography (petroleum ether: ethyl acetate = 10:1) gave 10.3 g of 2-(2-chloro-4-trifluoromethylphenyl)pyrim...
Embodiment 3
[0061] Embodiment three: the preparation of 2-(2,4-dichlorophenyl) pyrimidine
[0062] 2-(2,4-dichlorophenyl) pyrimidine adopts the following steps: 1. add 10 grams of 2-(4-chlorophenyl) pyrimidine, 300 mg of palladium acetate, 15 grams of additive trifluoro Copper acetate, 26 grams of calcium chloride, 100 milliliters of acetic acid, heated to 80 ° C, followed by thin-layer chromatography until the reaction raw material 2-(4-chlorophenyl) pyrimidine disappeared; ② after the reaction, use rotary evaporation Remove the solvent with an instrument, add saturated sodium bicarbonate solution to the system, extract the product with ethyl acetate, and remove the solvent with a rotary evaporator after drying to obtain a crude product; ③The crude product is subjected to column chromatography (petroleum ether: ethyl acetate=20 : 1) purification to obtain 11.4 g of 2-(2,4-dichlorophenyl)pyrimidine with a yield of 96%.
[0063] IR (KBr, cm -1 ): 3059, 3038, 1587, 1566, 1479, 1438, 1415,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com