Bifunctional catalyst and preparation method and application thereof
A dual-function catalyst and catalyst technology, applied in the direction of catalyst activation/preparation, preparation with chloride, chemical instruments and methods, etc., can solve the problems of deactivation, secondary pollution, catalyst poisoning, etc., and achieve no secondary pollution, Achieve recycling and long life effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
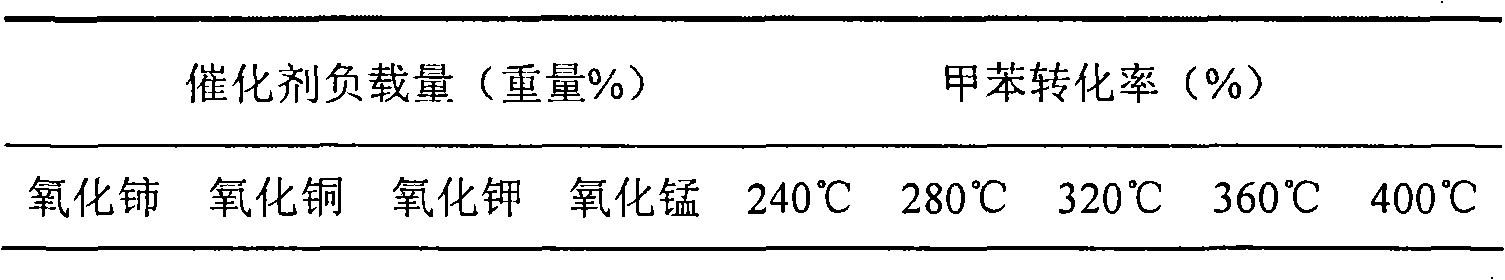
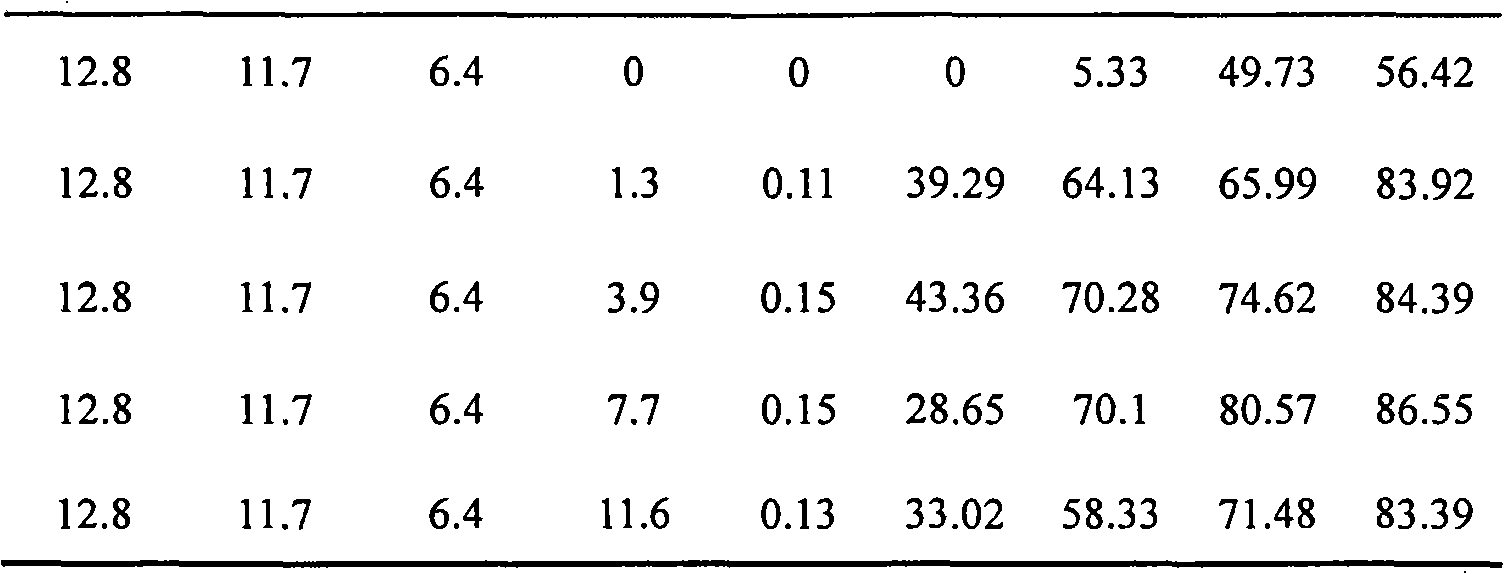
[0033] Commercially available 17.4g cerium nitrate (Ce(NO 3 ) 3 ·6H 2 (2), 5.5g Potassium Chloride KCl and 13.5g Cupric Chloride CuCl 2 2H 2 O is completely dissolved in distilled water, and based on the molar number of copper, manganese nitrate Mn(NO 3 ) 2 Aqueous solutions of 0g, 2.9g, 8.6g, 17.2g and 25.8g, mixed the obtained mixed metal ion solution with 54g of ReY molecular sieve and 20g of silica sol with a mass fraction of 30%, formed, and dried in the air at 120°C Drying under the atmosphere for 10h, then roasting at 550°C for 3h, to obtain catalysts with different manganese oxide loads, wherein the load of cerium oxide is 12.8% by weight (with the weight of ReY molecular sieve as a reference, the same below), and the load of potassium oxide is 6.4% by weight, and the loading amount of copper oxide is 11.7% by weight; the loading amount of manganese oxide is 0% by weight, 1.3% by weight, 3.9% by weight, 7.7% by weight, and 11.6% by weight; the loading amount of si...
Embodiment 2
[0035] Catalyst performance evaluation for deep oxidation combustion of organic impurities was carried out in an atmospheric pressure quartz tube reactor. The inner diameter of the reaction tube is 24 mm, the outer diameter of the thermocouple sleeve is 5 mm, and the amount of catalyst loaded is 2 grams. Toluene is extracted by a double plunger micropump and sent to the vaporization chamber for vaporization, mixed with oxygen, and then enters the reactor for deep oxidation combustion reaction. The volume concentration of toluene in the mixed gas is 0.3vol%, and the total gas flow rate is 500mL·min -1 , Airspeed 12000h -1. The reaction temperature ranges from 240 to 400°C. The catalysts prepared in Example 1 with different loadings of manganese oxide were used to measure the results of deep oxidation combustion of toluene, which are listed in Table 1.
[0036] Table 1 The results of deep oxidation combustion of toluene on catalysts with different manganese oxide loadings
...
Embodiment 3
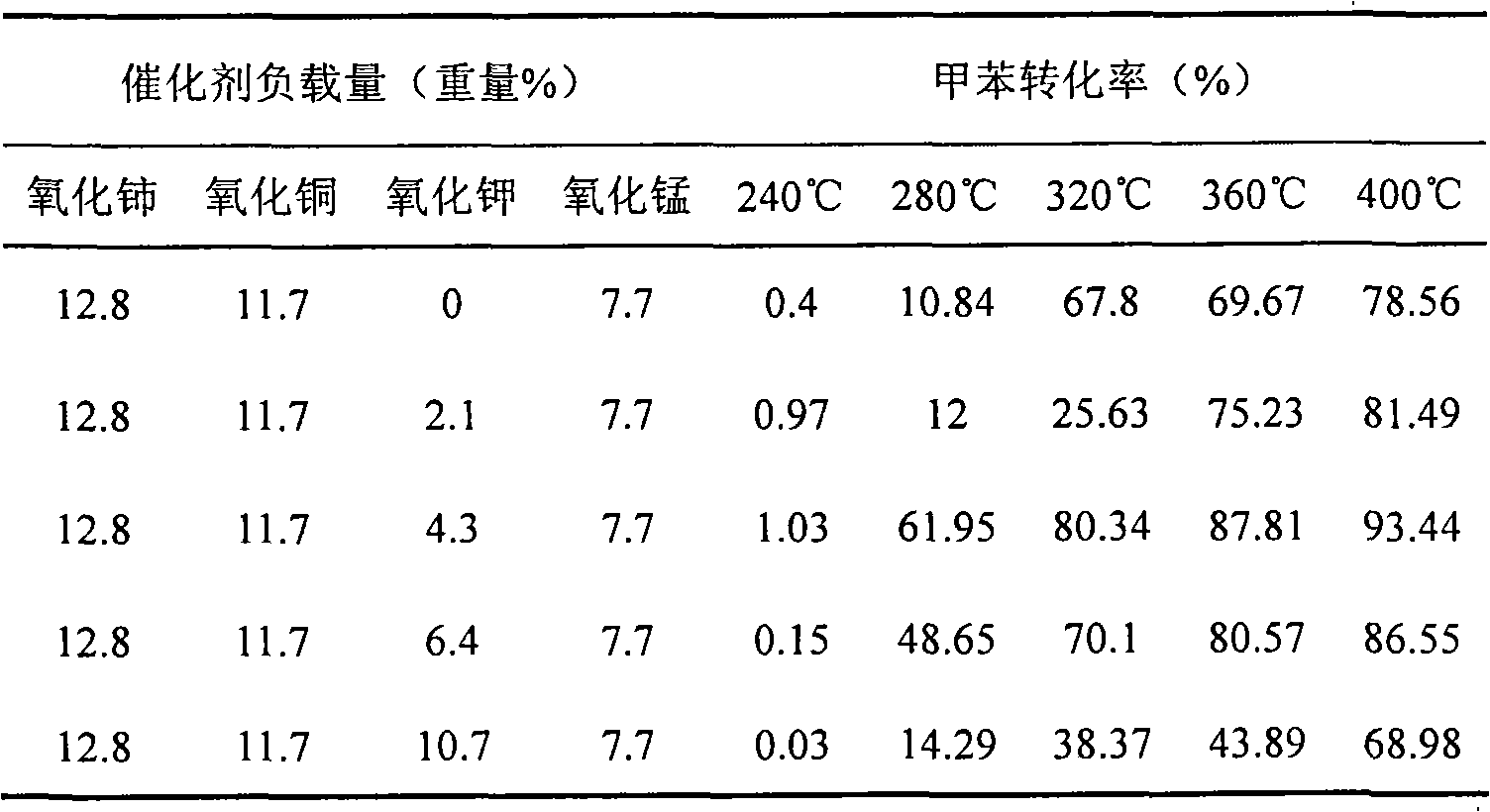
[0041] Commercially available 17.2g mass fraction is 50% manganese nitrate Mn(NO 3 ) 2 Solution, 13.5g copper chloride CuCl 2 2H 2 O and 17.4g Ce(NO 3 ) 3 ·6H 2 O is completely dissolved in distilled water, taking the molar number of copper as a basis, adding respectively potassium chloride 0g, 0.3, 0.6, 0.9, 1.5 potassium chloride 0g, 1.83g, 3.67g, 5.49g, 9.15g in the molar ratio of copper, the The resulting solution was mixed evenly with 50g of ReY molecular sieve and 20g of silica sol with a mass fraction of 30%, shaped, and dried in the air at 120°C for 10h, then calcined at 550°C for 3h to obtain different potassium oxide loadings catalyst, wherein the load of cerium oxide is 12.8% by weight (with the weight of ReY molecular sieve as a reference, the same below), the load of copper oxide is 11.7% by weight, and the load of manganese oxide is 7.7% by weight respectively; The loading amounts were 0 wt%, 2.1 wt%, 4.3 wt%, 6.4 wt%, and 10.7 wt%, respectively; the loadin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com