Medicament composition for treating colpitis symptoms and preparation method thereof
A composition and drug technology, which is applied in the direction of drug combination, medical formula, medical preparations containing active ingredients, etc., can solve the problems of easy loss of ointment, backward dosage form, strong water irritation, etc., and achieve environmental pollution reduction and hygienic use The effect of convenience and stable drug quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
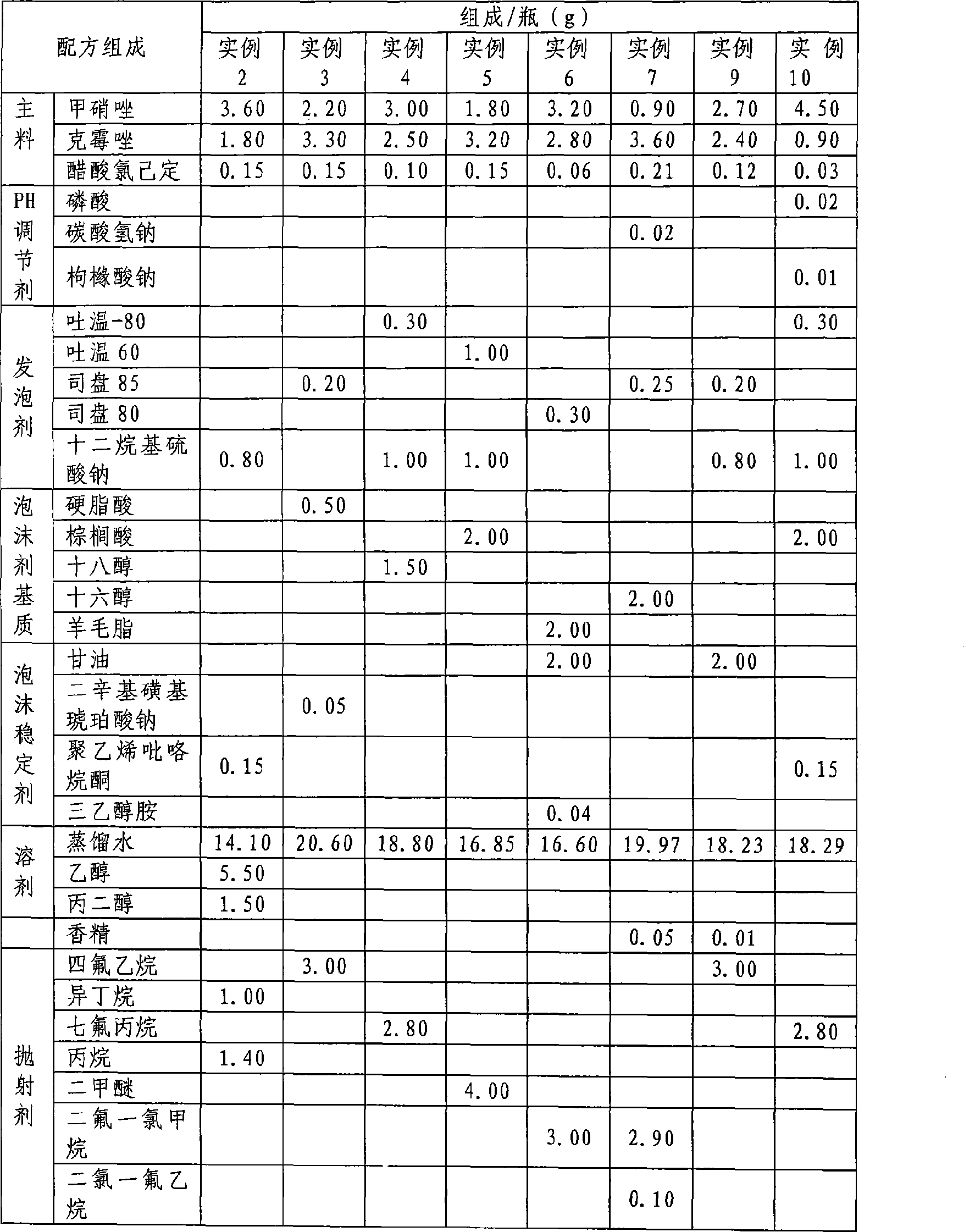
[0039] A foam for vaginal administration, the foam is composed of the following components, the proportion of each bottle is: metronidazole 3.00g, clotrimazole 2.00g, chlorhexidine acetate 0.10g, Tween-800.50 g, sodium lauryl sulfate 1.00g, water 13.10g, ethanol 6.00, glycerol 1.5g, tetrafluoroethane 2.80g, make solution type foaming agent, its preparation method is as follows:
[0040] (1) Metronidazole, clotrimazole, and chlorhexidine acetate of prescription quantity are stirred and dissolved in ethanol, glycerin, water mixed solvent;
[0041] (2) adding sodium lauryl sulfate of recipe quantity, Tween-80 stirs and makes it mix and dissolve;
[0042] (3) Check the content of the prepared medicinal liquid, and pour the qualified medicinal liquid into the pressure-resistant container;
[0043] (4) Cover the valve and seal it, and fill it with the prescribed amount of propellant.
[0044] It is also possible to directly assemble a mechanical pump that generates foam without us...
Embodiment 8
[0048] A foam for vaginal administration, the foam consists of the following ingredients, the proportion of each bottle is: Metronidazole 2.00g, clotrimazole 2.00g, chlorhexidine acetate 0.20g, Span-850.20 g, 0.80g sodium lauryl sulfate, 1.20g cetyl alcohol, 0.60g stearic acid, 0.01g essence, 19.99g water, 3.00g tetrafluoroethane, made into suspending foam, the preparation method is as follows :
[0049] (1) Weigh the prescription amount of cetyl alcohol, stearic acid, and Span-85 in a water bath and heat it to 50-70°C to dissolve it, and prepare it as liquid A for later use;
[0050] (2) Take 80% of the prescription amount of water, slowly heat it to 60-70 ° C, add the prescription amount of sodium lauryl sulfate, stir to dissolve, and it is B liquid; slowly add solution A to solution B while it is hot, and add While stirring, homogeneously emulsify to make emulsion;
[0051] (3) When the room temperature of the emulsion drops below 40°C, add micronized metronidazole, clotr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com