Insulin powder spray for lung inhalation and preparation method thereof
A technology of insulin and lung inhalation, applied in the field of pharmaceutical preparations, can solve problems such as the lack of conditions for developing mature dosage forms
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
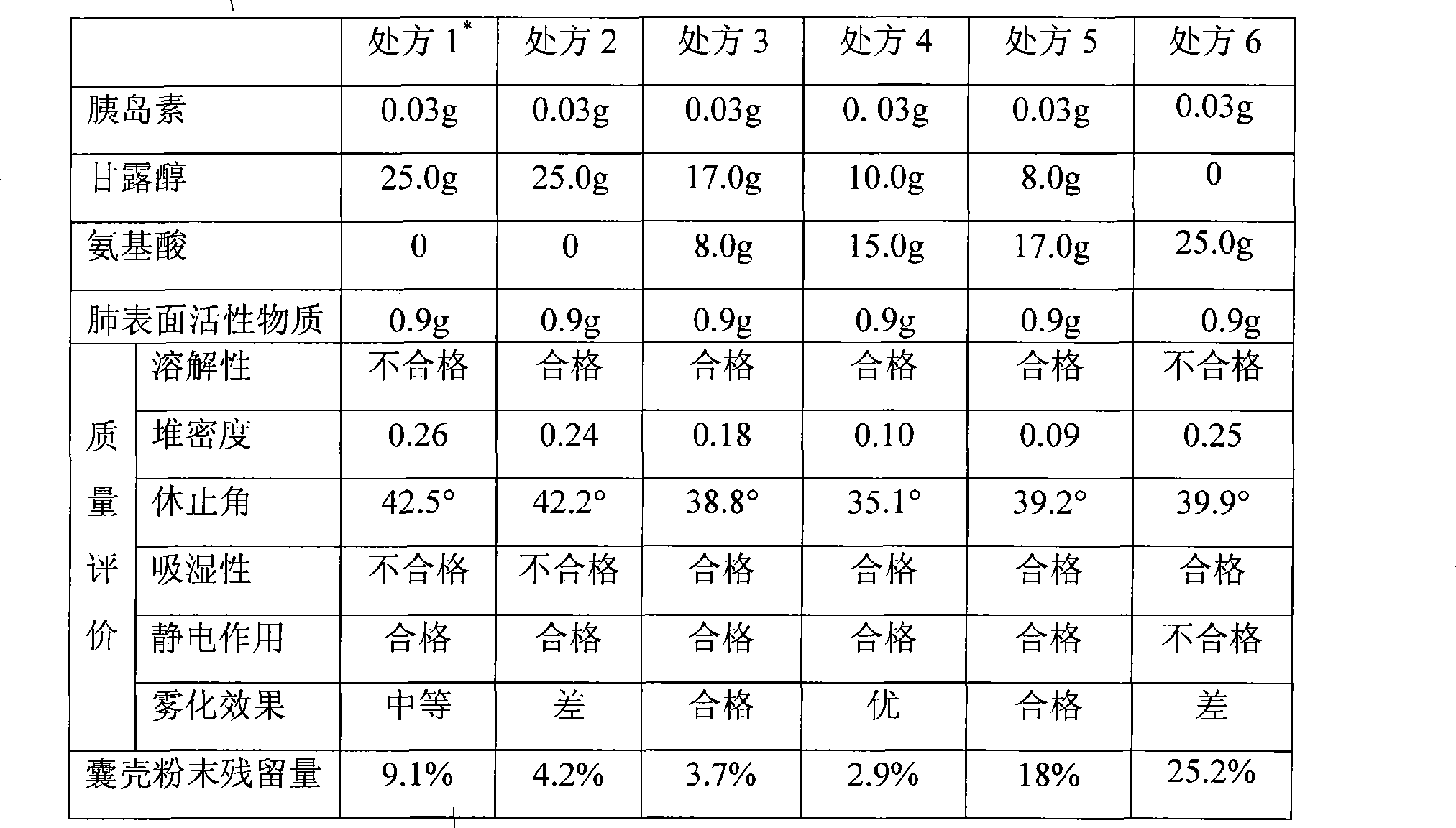
[0104] Take 3 g of recombinant human insulin and add an appropriate amount of hydrochloric acid with pH 2.0 to completely dissolve the recombinant human insulin to obtain solution 1. Add 72.0 g of mannitol and 18.0 g of leucine to about 2000 ml of water to dissolve to obtain solution 2; mix solution 1 and solution 2 evenly, add water to 3000 ml, and filter with a 0.2 μm microporous membrane to obtain solution 3. Weigh 18 g of artificial lung surfactant (among them: 14.4 g of egg yolk lecithin, 1.8 g of cetyl alcohol, and 1.8 g of tyloxapol) and dissolve it in absolute ethanol to obtain a lipid solution. Place the solution in a ground-mouthed round-bottom flask, and evaporate the organic solvent with a rotary evaporator at 100 rpm and reduced pressure on a constant temperature water bath at 50°C, so that film-forming materials such as phospholipids form a uniform lipid film at the bottom of the flask. . Use solution 3 to rotate and wash the membrane in a rotary evaporator to o...
Embodiment 2
[0106] Take 0.5 g of bovine insulin, add appropriate amount of hydrochloric acid with pH 2.0 to dissolve all the bovine insulin, and obtain solution 1. Add 15g of mannitol and 4g of leucine into about 400ml of water to dissolve to obtain solution 2; mix solution 1 and solution 2 evenly, add water to 800ml, and filter through a 0.2μm microporous membrane to obtain solution 3. Weigh 3.2 g of artificial lung surfactant (among them: 2.5 g of soybean lecithin, 0.4 g of cholesterol, and 0.3 g of tyloxapol) and dissolve it in absolute ethanol to obtain a lipid solution. Place the solution in a ground-mouthed round-bottom flask, and evaporate the organic solvent with a rotary evaporator at 100 rpm and reduced pressure on a constant temperature water bath at 50°C, so that film-forming materials such as phospholipids form a uniform lipid film at the bottom of the flask. . Use solution 3 to rotate and wash the membrane in a rotary evaporator to obtain a milky white crude lipid suspensio...
Embodiment 3
[0108]Take 0.5 g of recombinant human insulin and add an appropriate amount of NaOH solution with pH 8.0 to completely dissolve the recombinant human insulin to obtain solution 1. Add 12g of mannitol and 1.5g of threonine into about 200ml of water to dissolve to obtain solution 2; mix solution 1 and solution 2 evenly, add water to 800ml, and filter with a 0.2μm microporous membrane to obtain solution 3. Weigh 3.0 g of bovine lung surfactant extract (Beijing Shuanghe Modern Medical Technology Co., Ltd., National Pharmaceutical Approval H20052106), and suspend it with solution 3 to obtain a milky white thick lipid suspension. The suspension was subjected to high-pressure homogenization using a high-pressure homogenizer at a pressure of 900 bar to obtain a recombinant human insulin lipid suspension. The suspension was spray-dried under the following conditions: inlet temperature 110°C, outlet temperature 70°C, air flow rate 100%, nozzle air flow rate 800ml / min. The white powder ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com