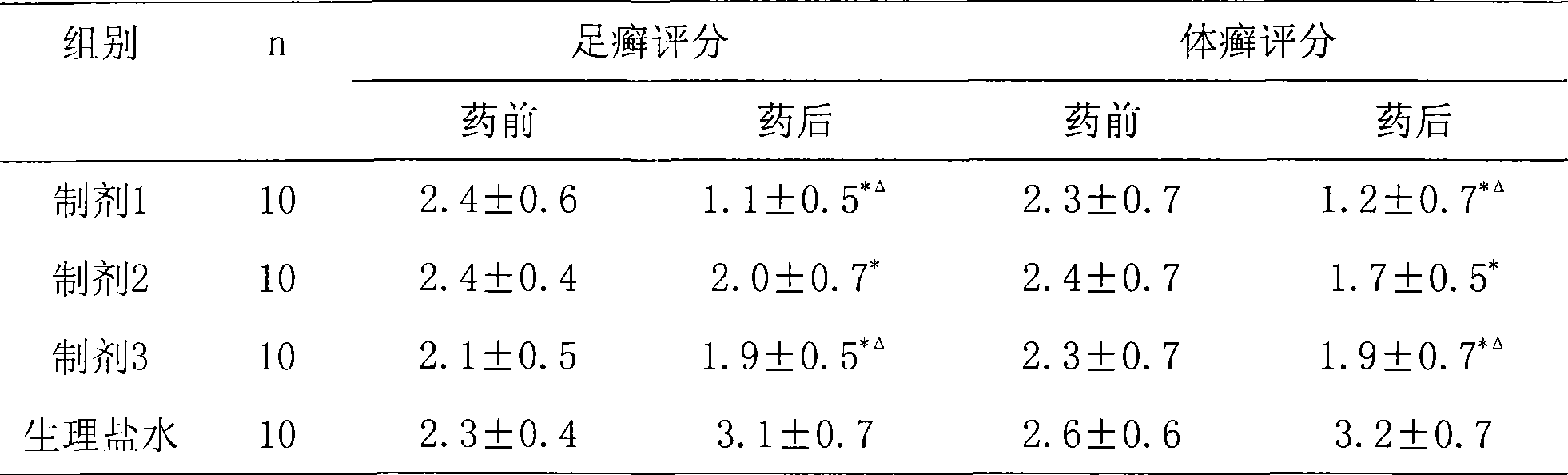
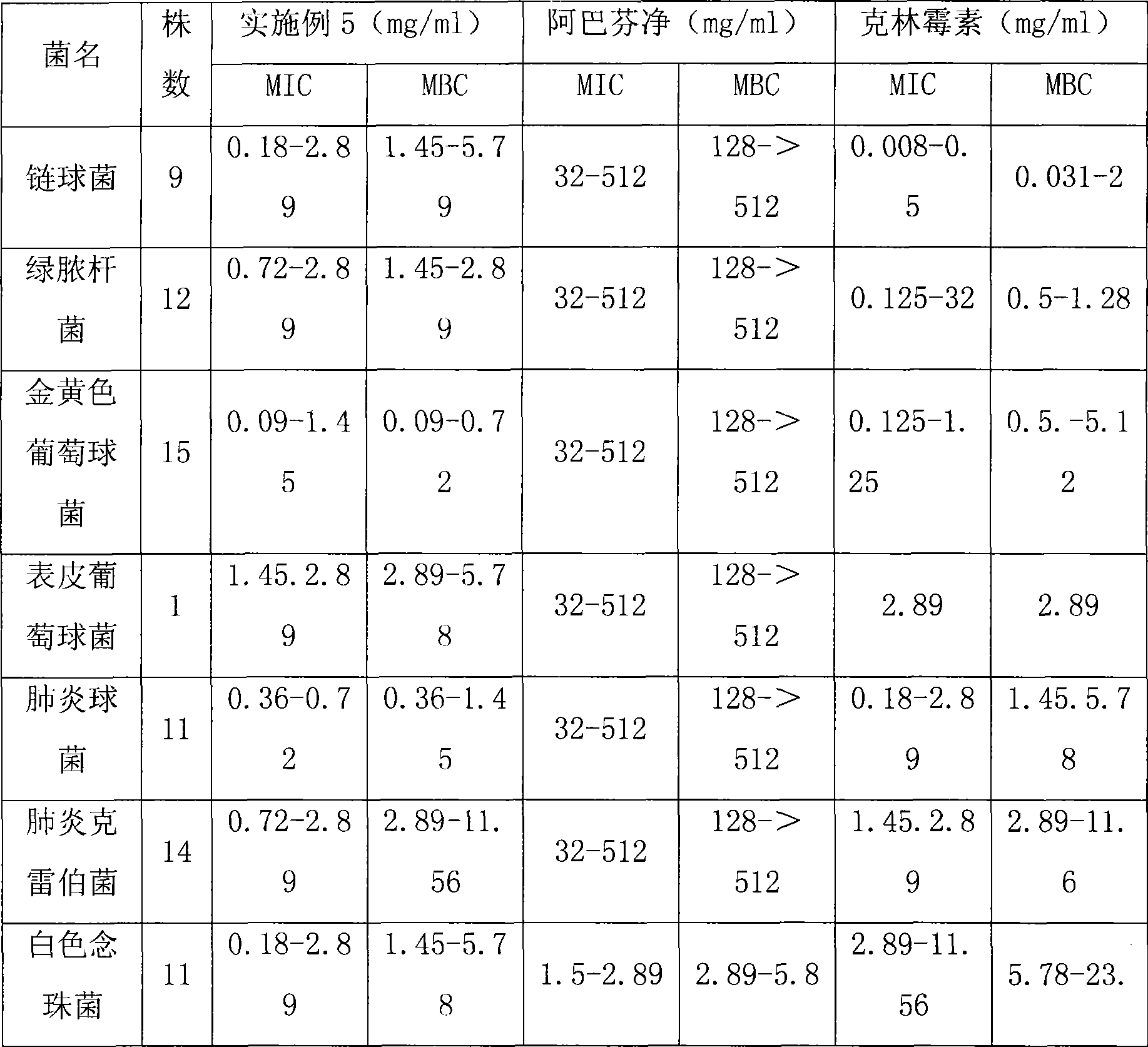Composite antibacterial drugs composition containing abafungin
The technology of an antibacterial drug, abafungin, applied in the field of preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Formula (cream): (1) Abafungin 10g, (2) Nystatin 4g, (3) Vaseline 10g, (4) Stearic acid 110g, (5) Liquid paraffin 60g, (6) Monostearin Glyceryl Acid Ester 35g, (7) Propylparaben 0.2g, (8) Stearyl Alcohol 50g, (9) Triethanolamine 4.5g, (10) Methylparaben 1.8g, (11) Di-tert-Butyl Hydroxyl Toluene 2g, (12) glycerin 40g, (13) sodium metabisulfite 10g, (14) ethylenediaminetetraacetic acid 1g, (15) add distilled water to 1000g.
[0024] Preparation method: put glycerin and sodium metabisulfite into a mortar and grind them evenly, then add abafungin and nystatin, and grind them evenly. This is ①; Vaseline, stearic acid, glyceryl monostearate, eighteen Mix alcohol, liquid paraffin, propylparaben, place in a water bath at 70-80°C to stir and dissolve, this is ②; mix methylparaben, di-tert-butylhydroxytoluene, ethylenediaminetetraacetic acid and water, Place in a water bath at 70-80°C to stir and dissolve, this is ③; add ① to ③, stir while adding, then add ②, stir until the emul...
Embodiment 2
[0026] Formula (cream): (1) Abafungin 15g, (2) Metronidazole 0.5g, (3) Lanolin 45g, (4) Acetylated monoglyceride 70g, (5) Liquid paraffin 60g, (6 ) cetyl alcohol 80g, (7) chlorhexidine gluconate 0.06g, (8) sodium sulfite 2g, (9) magnesium aluminum silicate 35g, (10) add distilled water to 1000g.
[0027] Preparation method: first mix lanolin, acetylated monoglyceride, liquid paraffin, and cetyl alcohol, put it in a water bath at 65-75°C, stir and dissolve, this is ①; add chlorhexidine gluconate, sodium sulfite, magnesium silicate Put aluminum and distilled water in a water bath at 70-80°C to stir and dissolve, this is ②; add ① while stirring ②, and set aside; mix abafungin and metronidazole, this is ③; add the above ③ in equal amounts to ①②In the mixed matrix, mix evenly, fill it up, and get it.
Embodiment 3
[0029] Recipe (suppository): (1) Abafungin 2.5g, (2) Clarithromycin 1.5g, (3) Glycerin gelatin 520g, (4) Vaseline 60g, (5) Triethanolamine 20g, (6) Dodecane Sodium sulfate 1g, (7) methyl paraben 1g, (8) propyl paraben 1g, (9) add distilled water to 1000g
[0030]Preparation method: mix glycerin gelatin and distilled water, and put it in a water bath at 50-60°C, stir, and dissolve it as ①; put petroleum jelly in a water bath at 50-60°C, dissolve it as ②; after ① and ② are dissolved , put ② into ①, stir evenly; mix abafungin with clarithromycin, triethanolamine, methylparaben, propylparaben and sodium lauryl sulfate evenly, then add to ①② mixture After stirring evenly, let it cool, and when it is close to solidification, pour it into a suitable pessary mold, overflow slightly, cool and solidify, scrape it flat, and take it out of the package.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com