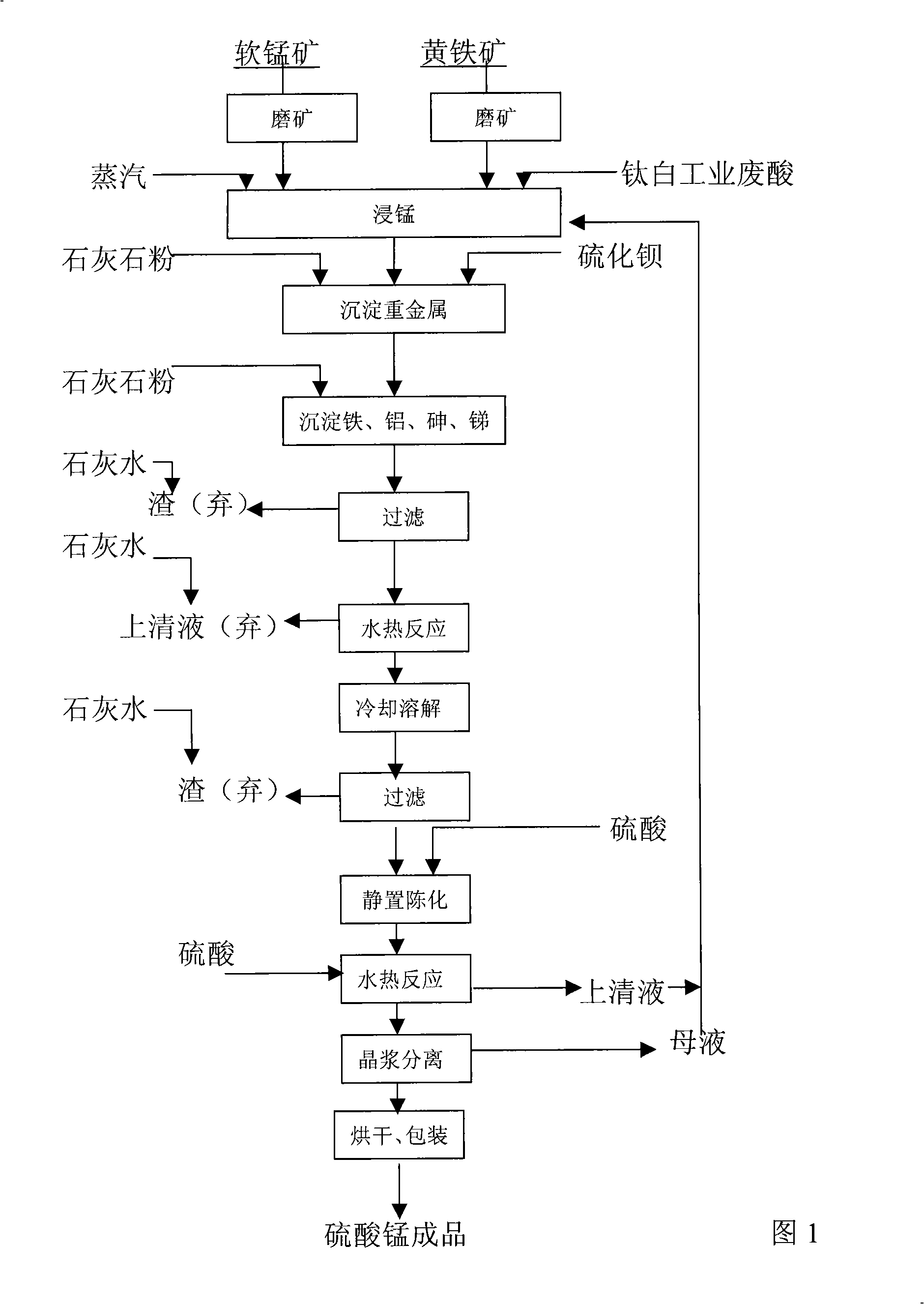
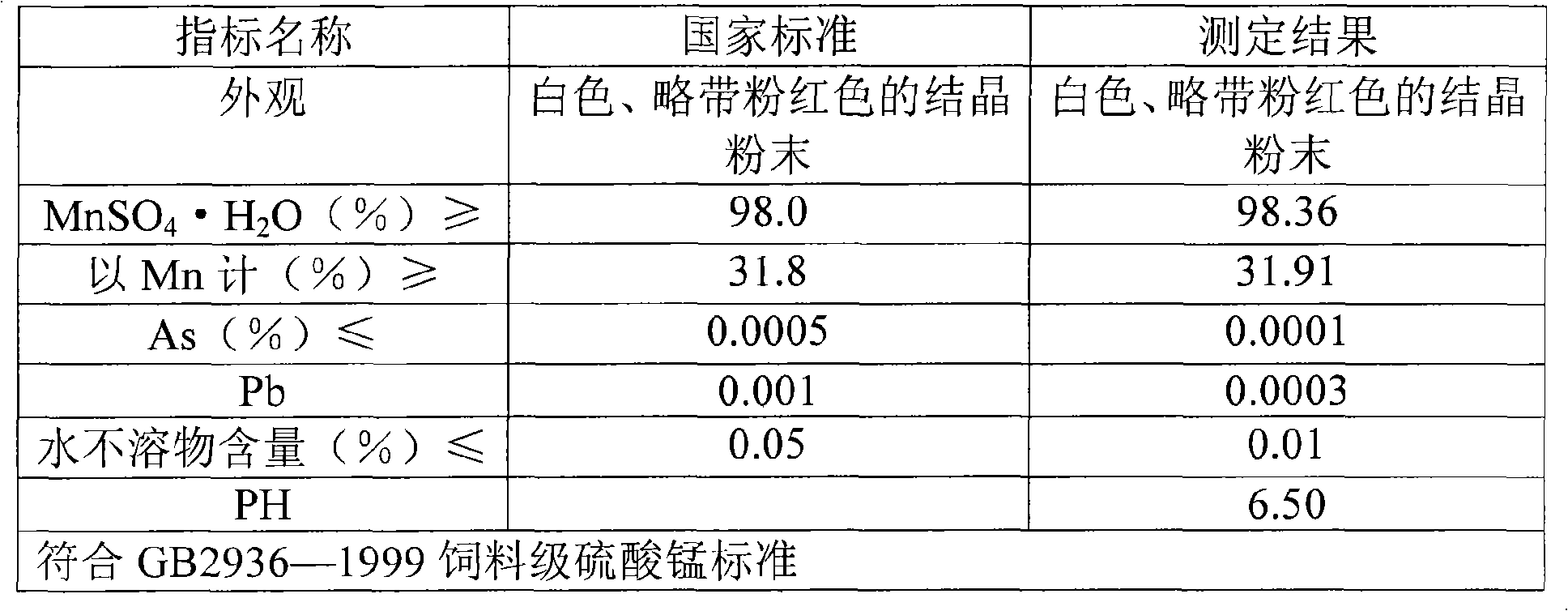
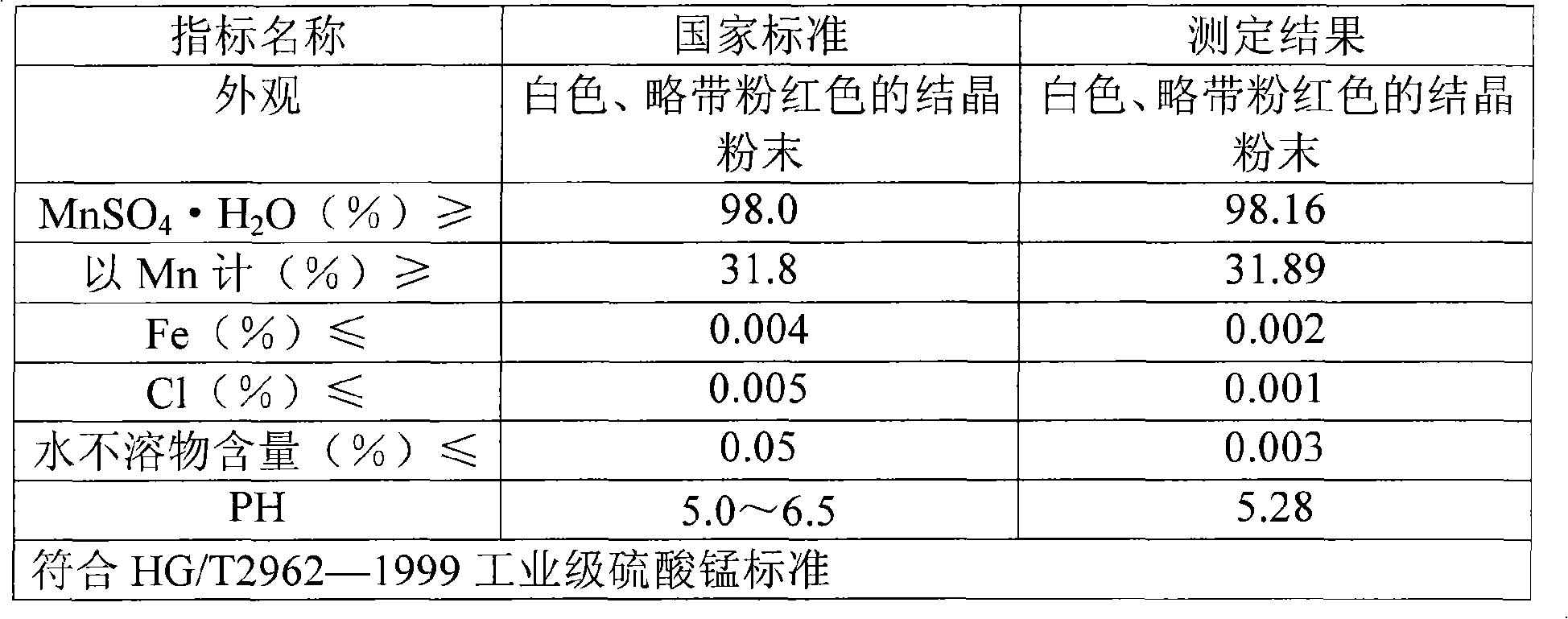
Method for producing manganese sulfate monohydrate crystal using pyrolusite and waste acid as raw material
A technology of manganese sulfate and pyrolusite, applied in the direction of manganese sulfate, etc., can solve the problems of waste of resources, low utilization rate of manganese, strong corrosion of equipment, etc., and achieve the effect of stable product quality, simple production process and high manganese recovery rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] ①The Guangxi Guipingsong soft manganese ore (MnO 2 ) (manganese content 16.10%), ground to -0.074mm, Yunfu pyrite (FeS 2 ) (sulfur content 30.51%), grind to -0.074mm, take titanium dioxide (TiO 2 ) plant waste acid {diluted H 2 SO 4 Contains FeSO 4 , ρ(H 2 SO 4 )=168g / L};
[0021] ②Take the above-mentioned titanium dioxide (TiO 2 ) industrial waste acid 0.39M 3 Inject into the manganese immersion barrel with stirring, and put -0.074mm fineness pyrolusite (MnO 2 ) 187Kg (dry basis) and -0.074mm fineness of pyrite (FeS 2 ) 30Kg (dry basis) powder into the manganese immersion tank, steamed and heated to 90°C for 2 hours of manganese immersion. At this point, the pH of the manganese (Mn) leaching solution was determined to be 1.05, and limestone (CaCO 3 ) powder to make the solution PH=3.51, add 0.62Kg of barium sulfide (BaS) to precipitate heavy metals, react for 20min, then add limestone (CaCO 3 ) powder to adjust the pH value to 5.2, the reaction time is 40mi...
Embodiment 2
[0027] ①The Guangxi Guipingsong soft manganese ore (MnO 2 ) (manganese content 17.2%), ground to -0.074mm, Yunfu pyrite (FeS 2 ) (sulfur content 30.51%), grind to -0.074mm, take Fuji County titanium dioxide (TiO 2 ) powder mill waste acid {(diluted H 2 SO 4 Contains FeSO4 ), ρ(H 2 SO 4 )=168g / L};
[0028] ②Take the above-mentioned titanium dioxide (TiO 2 ) industrial waste acid 0.36M 3 Inject into the manganese immersion barrel with stirring, and put -0.074mm fineness pyrolusite (MnO 2 ) 160Kg (dry basis) and pyrite (FeS 2 ) powder 30Kg (dry basis) into the manganese immersion bucket, soak manganese (Mn) at 95°C for 2 hours. At this time, the pH of the manganese (Mn) leaching solution was determined to be 1.43, and limestone (CaCO 3 ) powder to make the solution PH=2.53, add 0.6Kg of barium sulfide (BaS) to precipitate heavy metals, the reaction time is 30min, then add limestone (CaCO 3 ) powder to adjust the pH to 6.2, the reaction time is 35 minutes, precipitate i...
Embodiment 3
[0034] ①The Guangxi Guipingsong soft manganese ore (MnO 2 ) (manganese content 22.01%), ground to -0.074mm, Yunfu pyrite (FeS 2 ) (sulfur content 30.51%), grind to -0.074mm, take Fuji County titanium dioxide (TiO 2 ) Plant waste acid {(diluted H 2 SO 4 Contains FeSO 4 ), ρ(H 2 SO 4 )=168g / L}.
[0035] ②Take the above-mentioned titanium dioxide (TiO 2 ) industrial waste acid 0.42M 3 Inject -0.074mm fineness pyrolusite (MnO 2 ) 164Kg (dry basis) and pyrite powder (FeS 2 ) 36Kg (dry basis) into the manganese immersion barrel, and soak manganese (Mn) at 98°C for 2 hours. At this time, the pH of the manganese (Mn) leaching solution was determined to be 1.96, and limestone (CaCO 3 ) powder to make the solution PH = 3.0, add 0.6Kg barium sulfide (BaS) to react for 30 minutes to precipitate heavy metals [such as lead (Pb), zinc (Zn), cobalt (Co), nickel (Ni)]. Then adjust the pH value to 5.25, react for 40 minutes to precipitate iron (Fe), aluminum (Al), arsenic (As), anti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com