Amoxicillin sodium and sulbactam sodium for injection and preparation of freeze-dried injection thereof
A technology of amoxicillin sodium sulbactam sodium and amoxicillin sodium, applied in the field of separation and purification of amoxicillin sodium and sulbactam sodium, can solve the problem of not providing amoxicillin sodium sulbactam sodium freeze-dried powder injection and other issues to achieve the effect of avoiding sample loss, improving stability and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] A semi-preparative high-speed countercurrent chromatograph is used, equipped with a constant flow pump, a 15ml injection valve, a polytetrafluoroethylene column with a column volume of 200ml, and a UV ultraviolet detector. Mix chloroform, methanol, water, and ethyl acetate with a volume ratio of 2:1:1.5:1 in a separatory funnel, shake well, and let stand to separate layers. Take the upper layer solution (upper phase) as the stationary phase. The lower layer solution (lower phase) is the mobile phase. After ultrasonic degassing, first fill the entire column with the stationary phase, then turn on the high-speed countercurrent chromatograph, adjust the host speed to 1500rpm, and pump the mobile phase into the chamber at a flow rate of 1.5ml / min. After the dynamic equilibrium of the whole system is established, the amoxicillin sodium raw material is dissolved in the lower phase, and the sample is injected through the injection valve, and then according to the ultraviolet sp...
Embodiment 2
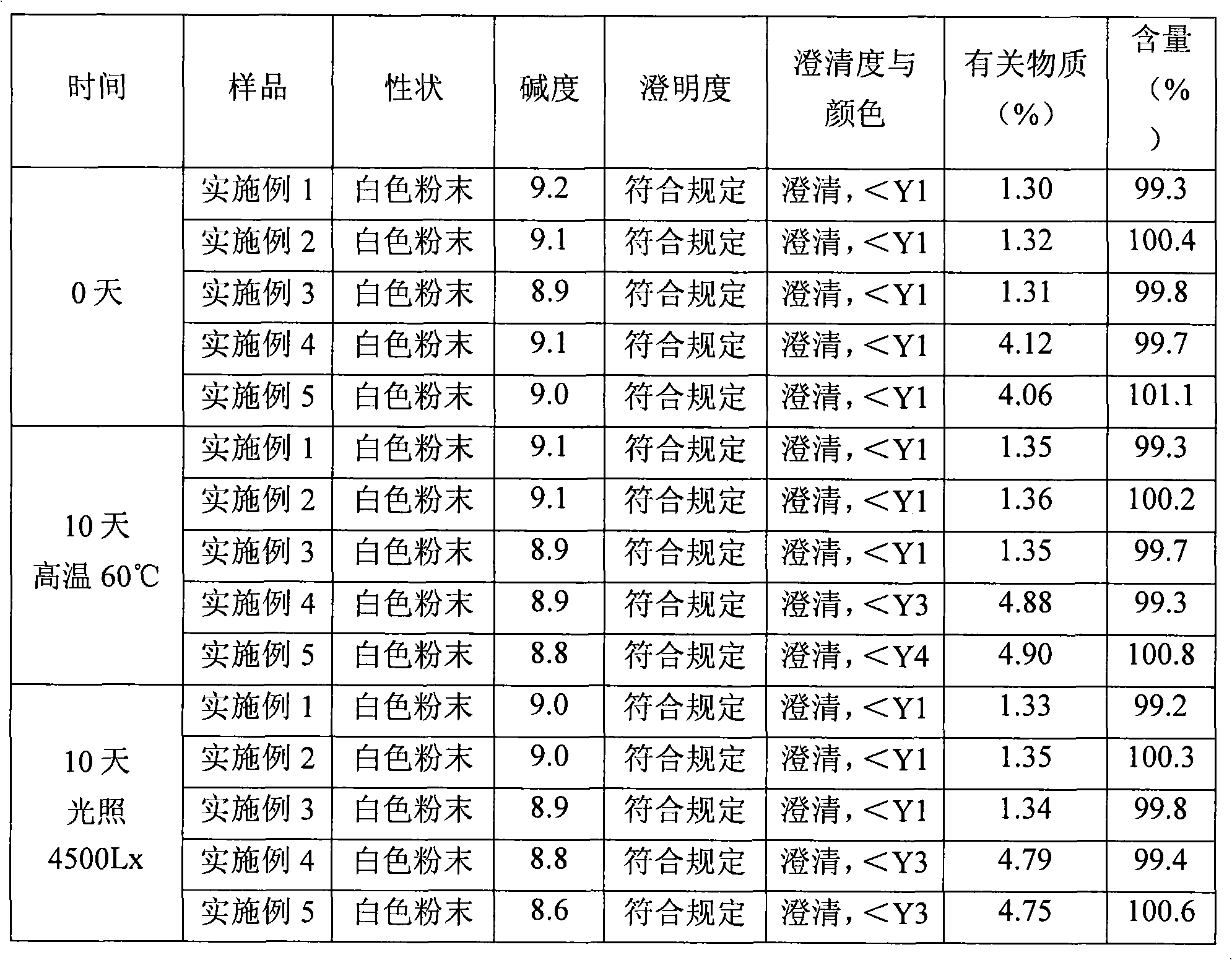
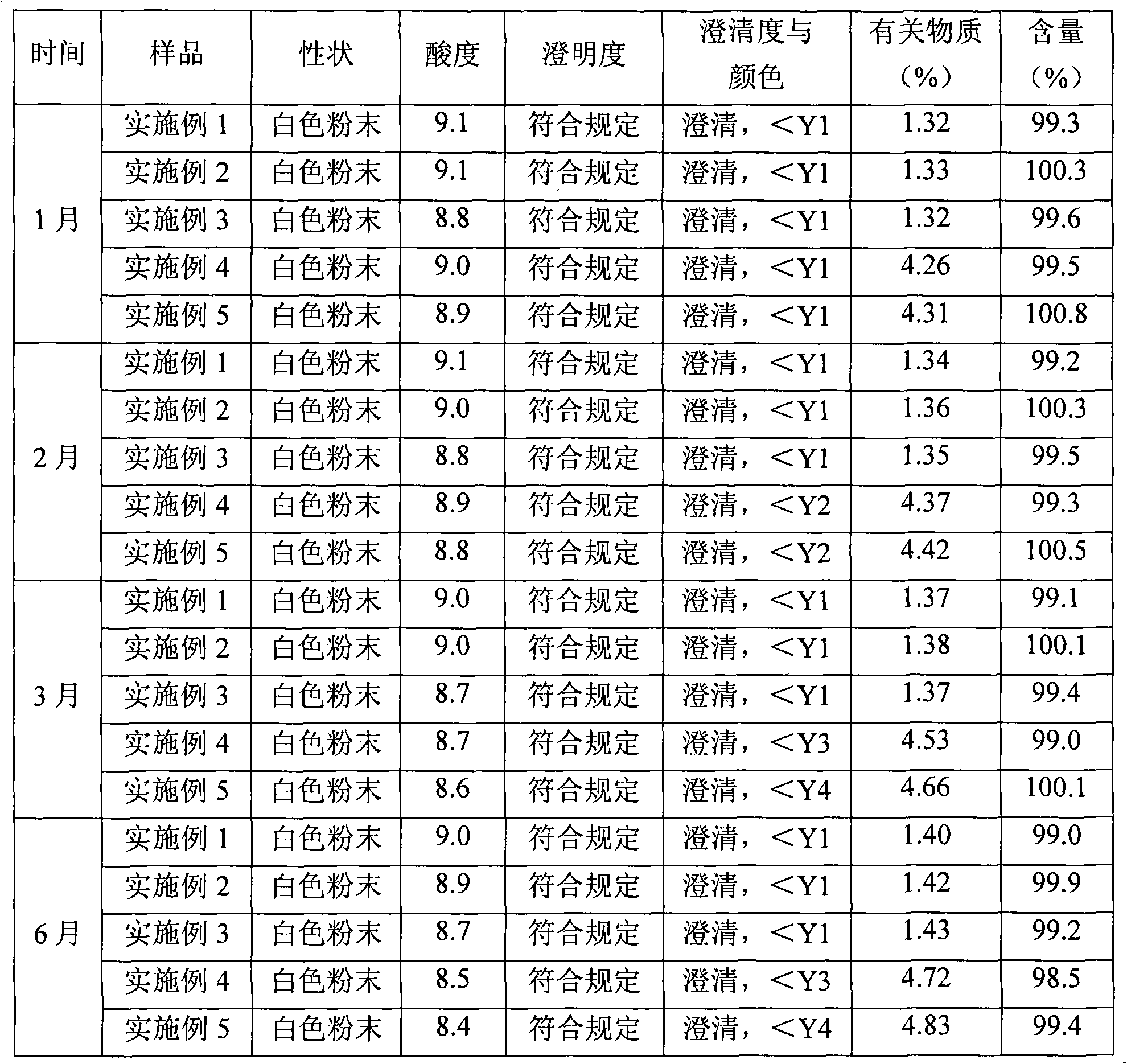
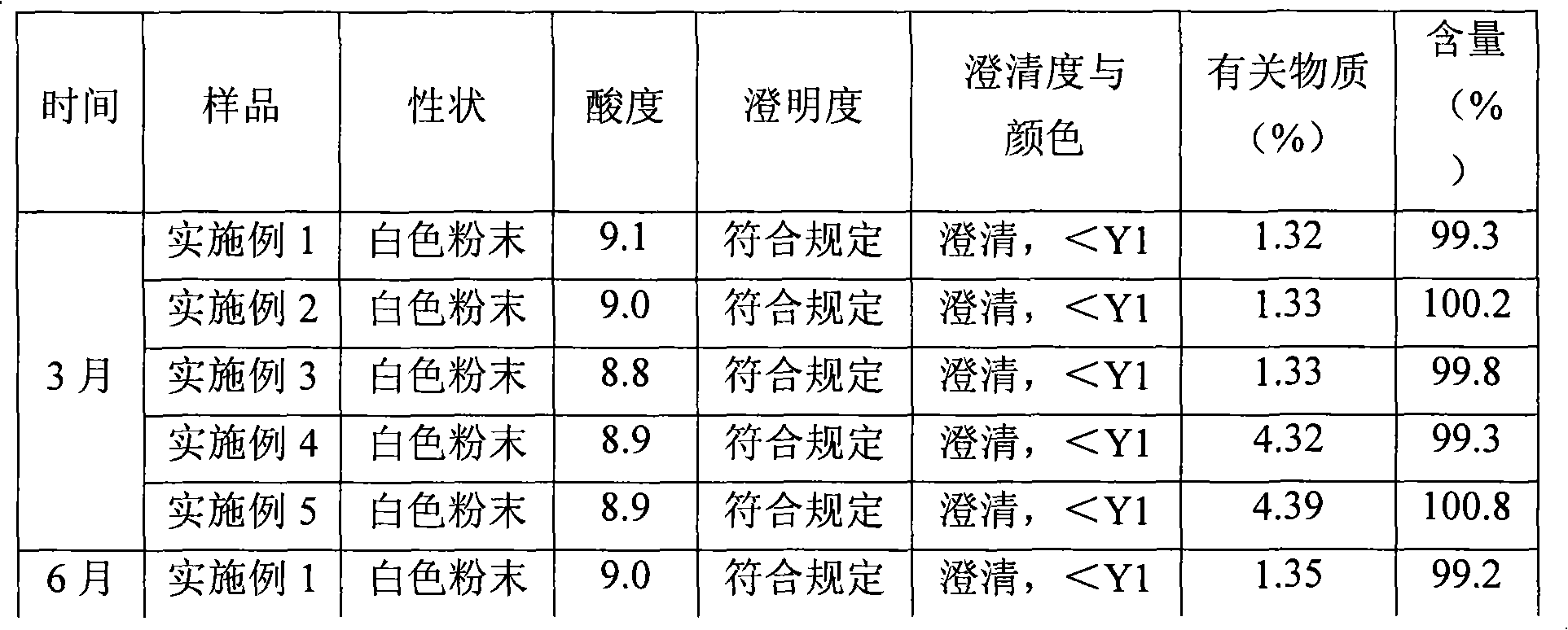
[0034] Purify amoxicillin sodium and sulbactam sodium raw materials according to the high-speed countercurrent chromatography operation steps of Example 1, the volume ratio of chloroform, methanol, water, and ethyl acetate is 1: 0.8: 1.2: 1, and analyzed by HPLC , the purity of amoxicillin sodium is 99.3%, the number of effective theoretical plates is 1870, the purity of sulbactam sodium is 99.2%, and the number of effective theoretical plates is 2230.
[0035] Pass the purified amoxicillin sodium and sulbactam sodium raw materials through 80-mesh sieve respectively, pulverize them, then mix them uniformly in a weight ratio of 2:1, and pack them under the condition of grade 100 in a sterile room to obtain amoxicillin sodium and sulbactam for injection Tan sodium powder injection, 3.0g per bottle.
Embodiment 3
[0037] Purify amoxicillin sodium and sulbactam sodium raw materials according to the high-speed countercurrent chromatography operation steps of Example 1, the volume ratio of chloroform, methanol, water, and ethyl acetate is 3: 2.5: 2: 1, and analyzed by HPLC , the amoxicillin sodium is 99.0%, the effective theoretical plate number is 2042, the purity of sulbactam sodium is 99.4%, and the effective theoretical plate number is 1955.
[0038]Pass the purified amoxicillin sodium and sulbactam sodium raw materials through a 100-mesh sieve and pulverize them, then mix them evenly at a weight ratio of 2:1, and pack them under the condition of grade 100 in a sterile room to obtain amoxicillin sodium and sulbactam for injection Tan sodium powder injection, 1.5g per bottle.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com