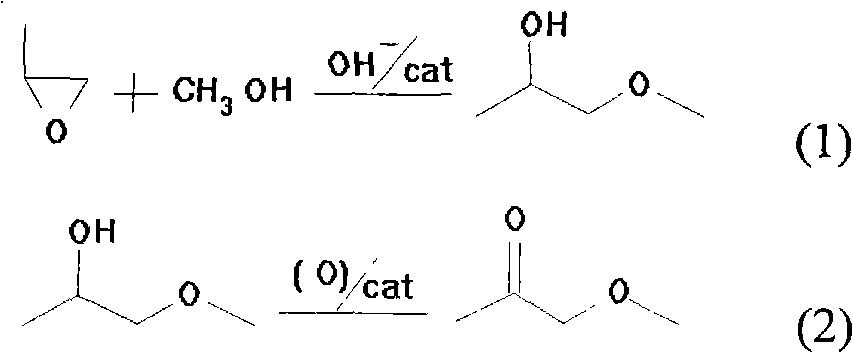
Method for preparing 1-methoxy-2-acetone with catalytic oxidation of 1-methoxy-2-propyl alcohol
A catalytic oxidation, methoxyl technology, applied in the preparation of carbon-based compounds, chemical instruments and methods, preparation of organic compounds, etc., can solve the complex reactor design and operation process, raw material 1-methoxy-2-propane Problems such as low alcohol conversion rate, to achieve the effects of easy operation, fast reaction rate and simple equipment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] Example 1: 18.0g (0.2mol) 1-methoxy-2-propanol and 0.312g (0.002mol) TEMPO catalyst were added to a 500mL polytetrafluoro-lined autoclave, magnetic stirring was started, and 0.138g dissolved in (0.002mol)NaNO 2 And 0.238g (0.002mol) KBr aqueous solution 1.2g, airtight reactor, fills with O 2 To a gauge pressure of 0.4 MPa, the temperature was raised to 80° C. and kept for 4 hours to complete the reaction. After the temperature was lowered and the pressure was carefully released, samples were taken for GC-MS analysis. The conversion rate of 1-methoxy-2-propanol was 89%, and the selectivity of the product 1-methoxy-2-propanone was 83%.
Embodiment 2
[0015] Example 2: 18.0g (0.2mol) 1-methoxy-2-propanol and 0.312g (0.002mol) TEMPO catalyst were added to a 500mL polytetrafluoro-lined autoclave, magnetic stirring was started, and 0.138g dissolved in (0.002mol)NaNO 2 And 0.238g (0.002mol) KBr, 0.168g of NaHCO 3 2.2 g of the aqueous solution, the reactor was sealed, filled with air to a gauge pressure of 0.7 MPa, and heated to 100 ° C for 4 hours to complete the reaction. After the temperature was lowered and the pressure was carefully released, samples were taken for analysis. The conversion rate of 1-methoxy-2-propanol was 85%, and the selectivity of 1-methoxy-2-acetone was 83%.
Embodiment 3
[0016] Example 3: 18.0g (0.2mol) 1-methoxy-2-propanol and 0.312g (0.002mol) TEMPO catalyst were added to a 500mL polytetrafluoro-lined autoclave, magnetic stirring was started, and 0.104g dissolved in (0.0015mol)NaNO 2 And 0.206g (0.002mol) NaBr, 0.168g of NaHCO 3 Add 1.2 g of aqueous solution of 1.2 g of liquid bromine, 36.0 g of 1,2-dichloroethane, seal the reaction vessel, fill it with air to 0.4 MPa (gauge pressure), raise the temperature to 80 ° C, keep the temperature for 4 hours to complete the reaction, evaporate After the dichloroethane solvent, sampling was carried out for gas chromatography analysis, the conversion rate of 1-methoxyl-2-propanol was 90%, and the selectivity of product 1-methoxyl-2-propanone was 67%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com