Water-soluble arteannuin derivative and preparation method thereof
A water-soluble technology of artemisinin derivatives, applied in pharmaceutical formulations, drug combinations, allergic diseases, etc., can solve the problems of low oral bioavailability, short half-life, damage to the central nervous system, etc., to increase bioavailability , reduce toxic and side effects, and have novel synthetic ideas
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
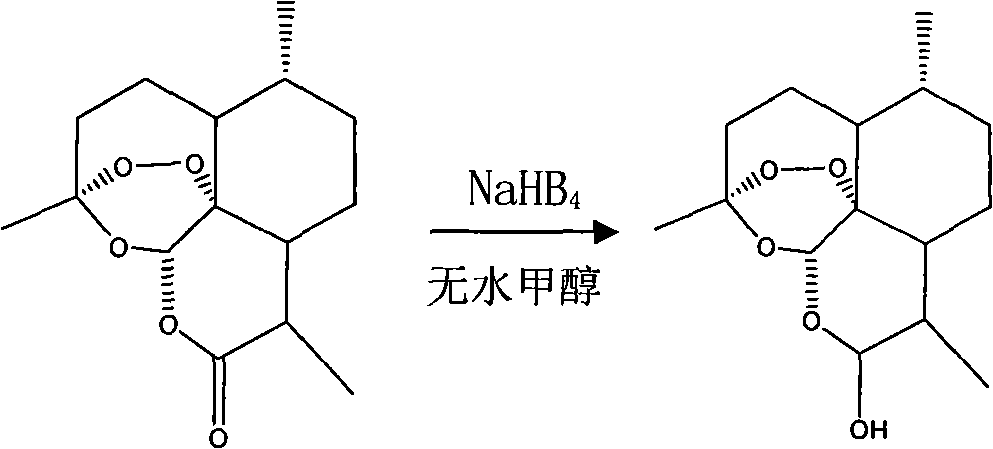
[0065] Embodiment 1: the reduction of artemisinin
[0066] Add 2.81g (0.01mol) artemisinin, 0.454g (0.012mol) NaHB 4 And 10mL of anhydrous methanol stirred at room temperature for 24h. The filtrate was distilled under reduced pressure to recover methanol. The residue was dissolved in 250 mL of water, then extracted three times with 50 mL of ethyl acetate, the organic layer was washed with a small amount of water, and the water washings and the water layer were combined. It was extracted with ethyl acetate, and the organic layers were combined. Evaporate solvent, dry product-dihydroartemisinin (see figure 1 );
Embodiment 2
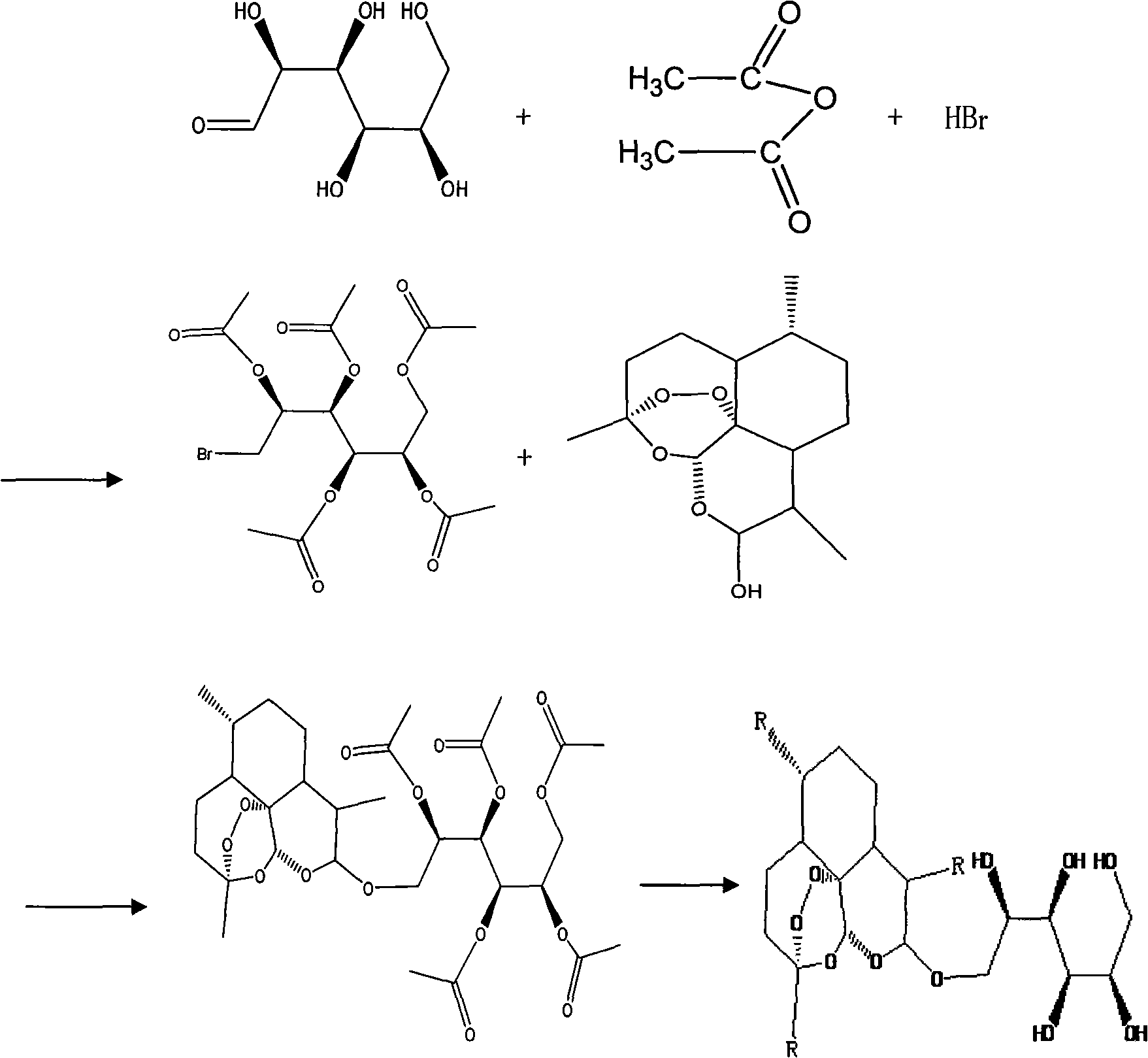
[0067] Example 2. Preparation of bromomannose per acetylpyranoside
[0068] Take 2.00 grams of D-mannose and add it to a three-neck flask containing 10 mL of acetic anhydride suspension (wherein acetic anhydride protects the hydroxyl on the sugar molecule to make it a stable ester), add 135 mg of iodine, and stir the reaction with magnetic force at room temperature Until the reaction system is brown and transparent (indicating that the full acetylation reaction is completed for about 5-30min), then the reaction mixture is diluted with 50mL of dry dichloromethane, and 14mL of 40% hydrogen bromide in glacial acetic acid solution is added under ice-cooling (addition of bromine Hydrogen, so that the sugar molecule with a -Br). After the addition, stir the reaction at room temperature until the reaction is detected by TCL (about 1-6h). The reaction mixture was diluted with 150 mL of dichloromethane, washed successively with ice water (60 mL×2), saturated sodium bicarbonate solutio...
Embodiment 3
[0069] Example 3. Preparation of D-mannose-artemisinin:
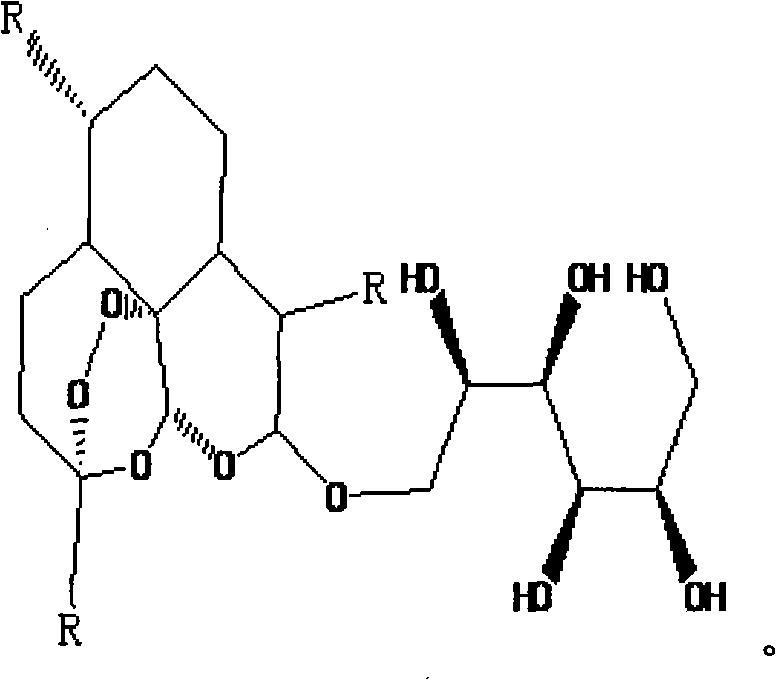
[0070] see figure 2 . Add the artemisinin reduction product dihydroartemisinin 1.354g that embodiment 1 obtains in the 100mL three-neck bottle that magnetic stirrer is housed, tetrabutylammonium hydrogensulfate 0.2g (tetrabutylammonium hydrogensulfate is a phase transfer catalyst ), distilled water 50mL, add potassium hydroxide 0.134g, adjust the pH of the solution to be 8-9. After stirring and refluxing for 4 hours, add 1.62 g of peracetylpyranobromomannose described in Example 2 to the reaction system, condense and reflux for 5 hours, cool, filter, and recrystallize the filter cake with diethyl ether and petroleum ether to obtain the present invention. Water-soluble artemisinin derivative - D-mannose - artemisinin.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com