Nitrofurans medicament metabolite residue ELISA kit and use method
An enzyme-linked immunosorbent assay, an enzyme-linked immunosorbent assay technology, applied in the field of enzyme-linked immunosorbent assay, can solve the problems of prolonged detection time, difficult to apply in practice, single drug metabolite residue detection, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
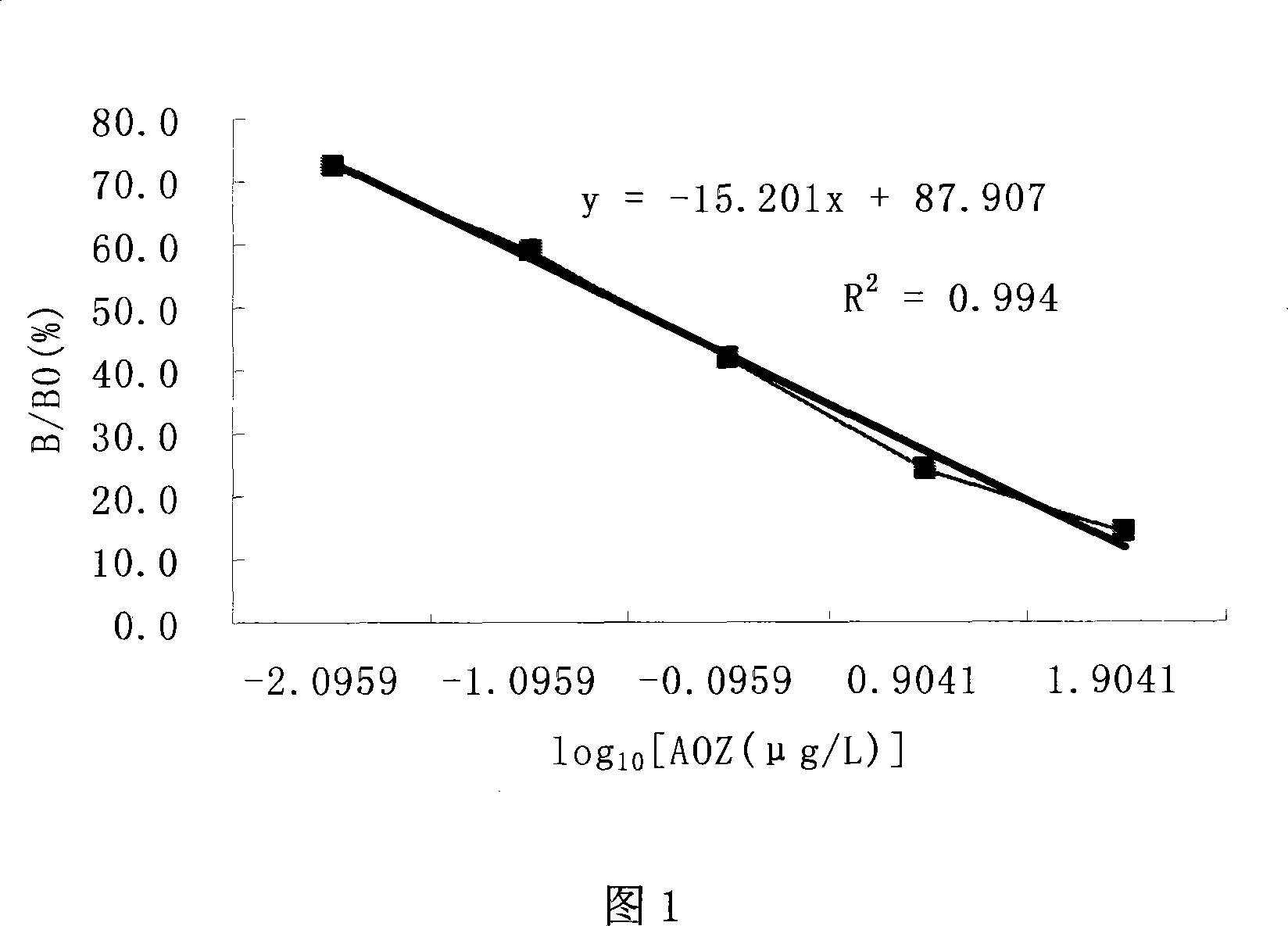
Image
Examples
Embodiment 1
[0096] The preparation of embodiment 1 antigen
[0097] (1) Preparation of nitrofuran metabolite hapten:
[0098] a. Synthesis of metabolite AOZ hapten
[0099] 3-Amino-2-oxazolidinone (AOZ) reacts with p-aldehyde benzoic acid to form a hapten with a carboxyl group.
[0100] b. Synthesis of metabolite AMOZ hapten
[0101] 3-Amino-5-morpholinomethyl-2-oxazolidinone (AMOZ) reacts with p-aldehyde benzoic acid in water to generate a hapten with a carboxyl group.
[0102] c. Synthesis of metabolite SC hapten
[0103] Reduce the nitro group of semicarbazide (SC) to amino group to generate hapten with amino group.
[0104] d. Synthesis of metabolite AH hapten
[0105] Amino-hydantoin (AH) reacts with m-aldobenzoic acid in water to form a carboxyl-bearing hapten.
[0106] (2) Preparation of nitrofuran metabolite antigen:
[0107] a. Dissolve 50 μmol / L nitrofuran metabolite hapten in 1 mL of DMF, then add equimolar DCC and NHS to the solution, let it react overnight at room temp...
Embodiment 2
[0111] The preparation of embodiment 2 antibody
[0112] Preparation of mouse monoclonal antibody against nitrofuran metabolites:
[0113] Animal immunization procedure: Balb / c mice were used as immunized animals, nitrofuran metabolite hapten and bovine serum albumin conjugate were used as immunogen, and the immunization dose was 60 μg / mouse. Freund's complete adjuvant was mixed to make an emulsifier, injected intraperitoneally, and the same dose of immunogen plus an equal amount of Freund's incomplete adjuvant was mixed and emulsified at intervals of 3 weeks, and the booster was given once. splenocytes.
[0114] Cell fusion and cloning: Splenocytes from immunized Balb / c mice were fused with SP2 / 0 myeloma cells at a ratio of 4:1. Cell supernatants were measured by indirect competitive enzyme-linked immunosorbent assay, and positive wells were screened. The positive wells were cloned by microcloning until a hybridoma cell line stably secreting the monoclonal antibody was obta...
Embodiment 3
[0117] The extraction of embodiment 3 horseradish peroxidase or alkaline phosphatase
[0118] 1. Extraction of horseradish peroxidase
[0119] a. Water extraction: take by weighing 20 kilograms of washed fresh horseradish or horseradish skin, cut into small pieces, and mince in a pulverizer. Add 10 kg of water to the crushed slag slurry, stir and extract at low temperature for 8 hours, centrifuge at 3000 rpm for 10 minutes, and collect the supernatant.
[0120] b. Fractional separation of ammonium sulfate: add 226 grams of ammonium sulfate powder per liter of filtrate, stir while adding, put overnight at room temperature. The next day, draw the supernatant, and then add 258 grams of ammonium sulfate powder per liter of supernatant, and stir as you add it. After the ammonium sulfate is completely dissolved, put it in a cold room overnight. The next day, the supernatant was sucked off, and the precipitated part was centrifuged at 13,000 rpm for 20 minutes in a refrigerated cen...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com