Sirolimus lipidosome freeze-dried acanthopanax powder and technique of preparing the same
A technology for sirolimus liposome and freeze-dried powder injection, which is applied in the field of sirolimus liposome freeze-dried powder injection and its preparation technology, can solve the problems of low bioavailability of sirolimus and the like, and achieves prolonged blood circulation. Circulation time, improved efficacy, improved bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
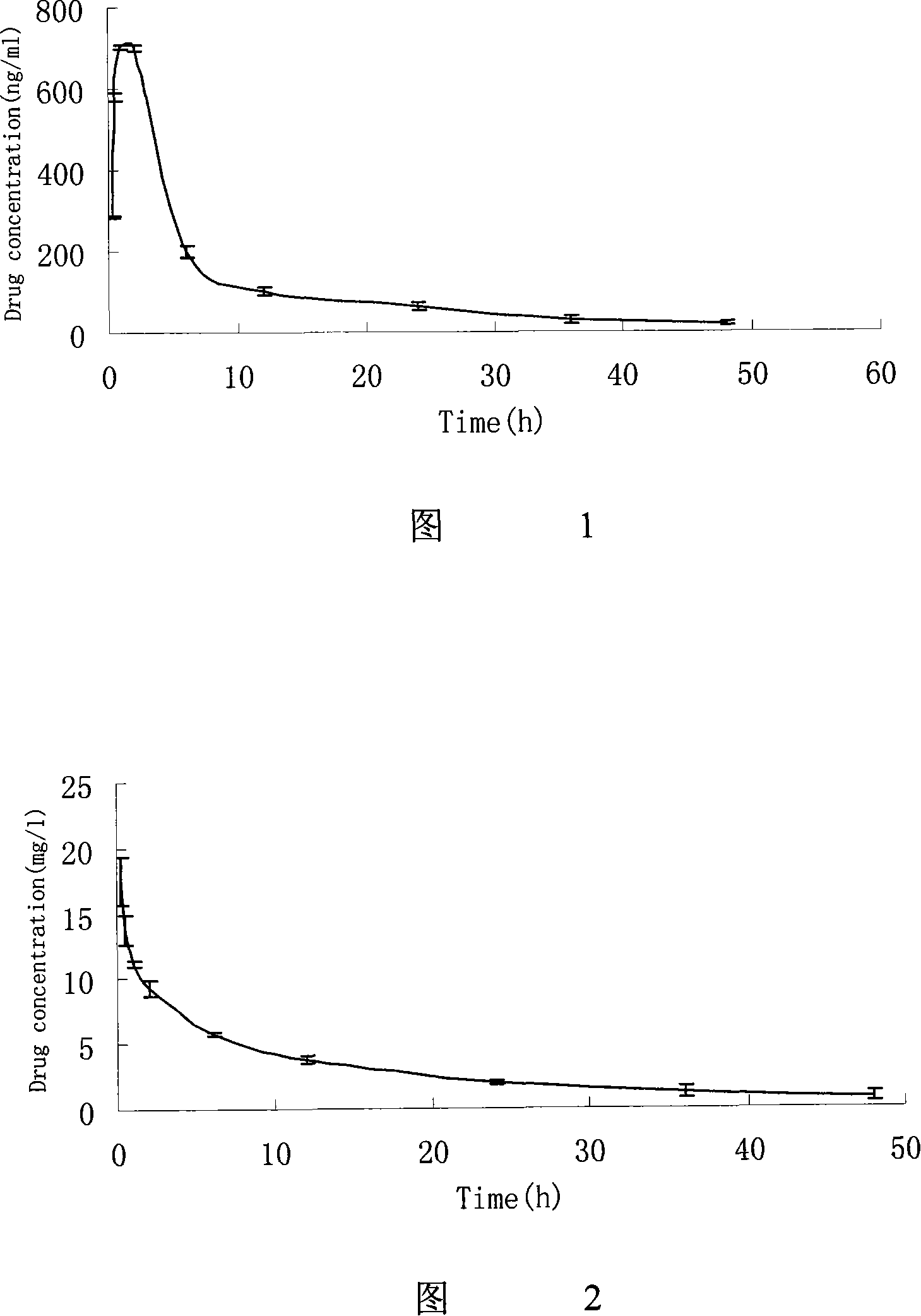
Image
Examples
Embodiment 1
[0056] Prepare raw materials with the following weights or volumes:
[0057] Sirolimus 500mg
[0058] Soy Lecithin 2.3g
[0059] Cholesterol 1g
[0060] Vitamin E 21mg
[0061] Phosphate buffer (p H7.4, 0.01mol / L) 300ml
[0062] Mannitol 100g
[0063] Chloroform 400ml
[0065] Water for injection 1000ml
[0066] Dissolve sirolimus, phospholipids, cholesterol, and vitamin E in chloroform according to the weight ratio of raw materials in the formula and mix uniformly; remove the organic solvent in the solution under reduced pressure with a rotary evaporator (temperature is not higher than 40° C.), and A lipid film is formed on the glass bottle wall; add 300ml of isotonic phosphate buffer (pH6-7.4, 0.01mol / L, containing 0.9% Nacl), stir for 30min, and place it at room temperature for 2h to make the film imbibition; Stir at room temperature for 2 hours to obtain an aqueous liposome suspension; the obtained aqueous liposome suspension is processe...
Embodiment 2
[0068] Prepare raw materials with the following weights or volumes:
[0069] Sirolimus 1000mg
[0071] Distearoylphosphatidylcholine 5g
[0072] Cholesterol 7g
[0073] Vitamin E 100mg
[0074] Phosphate buffer (p H7.4, 0.01mol / L) 300ml
[0075] Mannitol 150g
[0076] Chloroform 500ml
[0078] Water for injection 1000ml
[0079] Sirolimus, phospholipid, cholesterol, vitamin E are dissolved in 2: 1 chloroform-ethanol mixed solvent according to the weight ratio of raw material in the formula and mix homogeneously; With rotary evaporator, the organic solvent in the solution is removed under reduced pressure (temperature not higher than 40°C), a lipid film is formed on the glass bottle wall; add 300ml of isotonic phosphate buffer (p H6-7.4, 0.01mol / L, containing 0.9% Nacl), stir for 30min, and place at room temperature 2h, make film imbibition; Stir at room temperature 2h again, obtain liposome water suspension; 0...
Embodiment 3
[0081] Prepare raw materials with the following weights or volumes:
[0082] Sirolimus 1000mg
[0083] Soy Lecithin 3g
[0084] Distearoylphosphatidylcholine 5g
[0085] Cholesterol 7g
[0086] Vitamin E 100mg
[0087] Phosphate buffer (pH7.4, 0.01mol / L) 300ml
[0088] Mannitol 150g
[0089] Chloroform 500ml
[0091] Water for injection 1000ml
[0092] Sirolimus, phospholipid, cholesterol, vitamin E are dissolved in 2: 1 chloroform-ethanol mixed solvent according to the weight ratio of raw material in the formula and mix homogeneously; With rotary evaporator, the organic solvent in the solution is removed under reduced pressure (temperature Not higher than 40°C), a lipid film is formed on the glass bottle wall; add 300ml of isotonic phosphate buffer (pH6-7.4, 0.01mol / L, containing 0.9% Nacl), stir for 30min, and place at room temperature for 2h , to make the film imbibition; then stirred at room temperature for 2 hours to obtain an aqueous li...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com