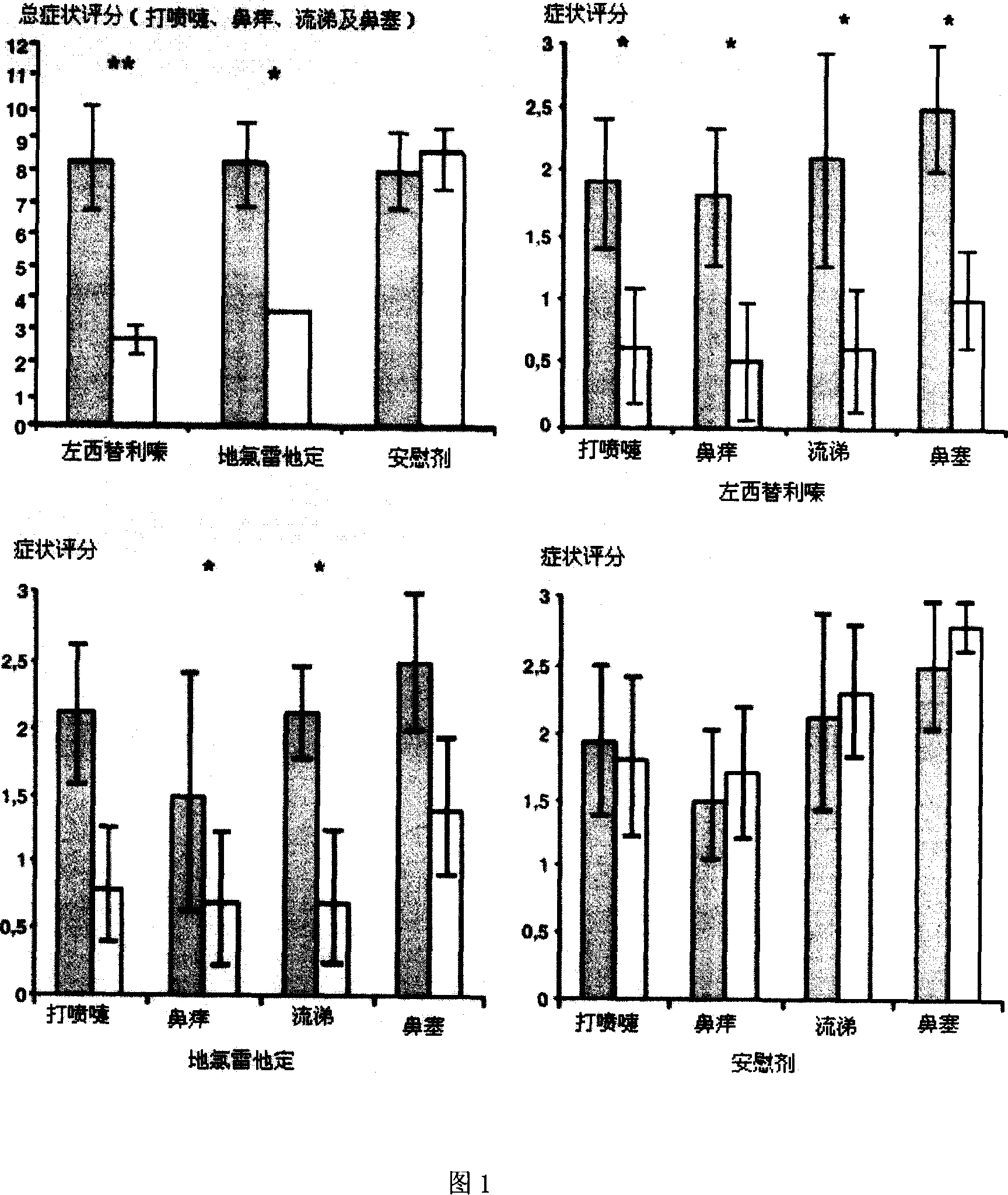
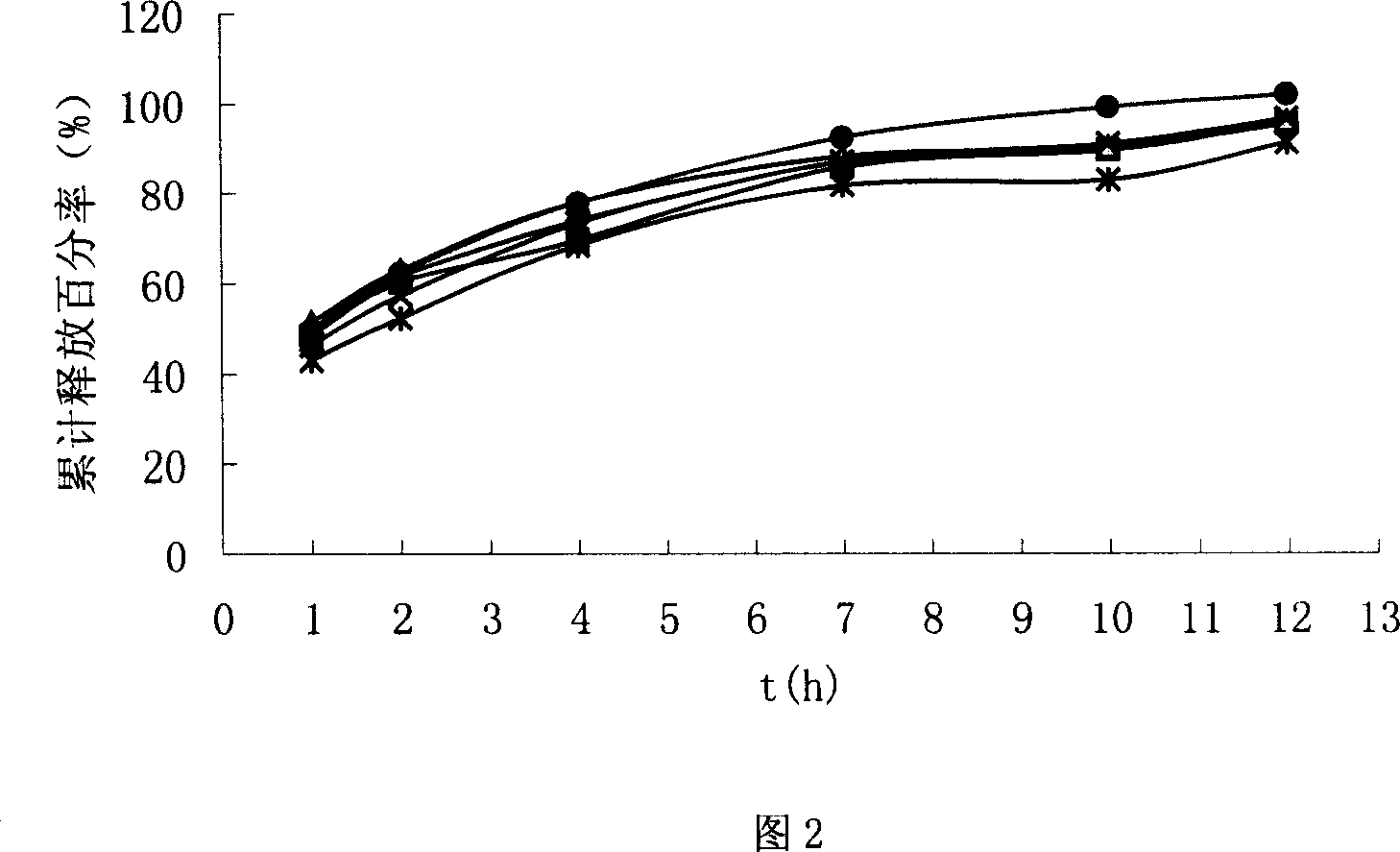
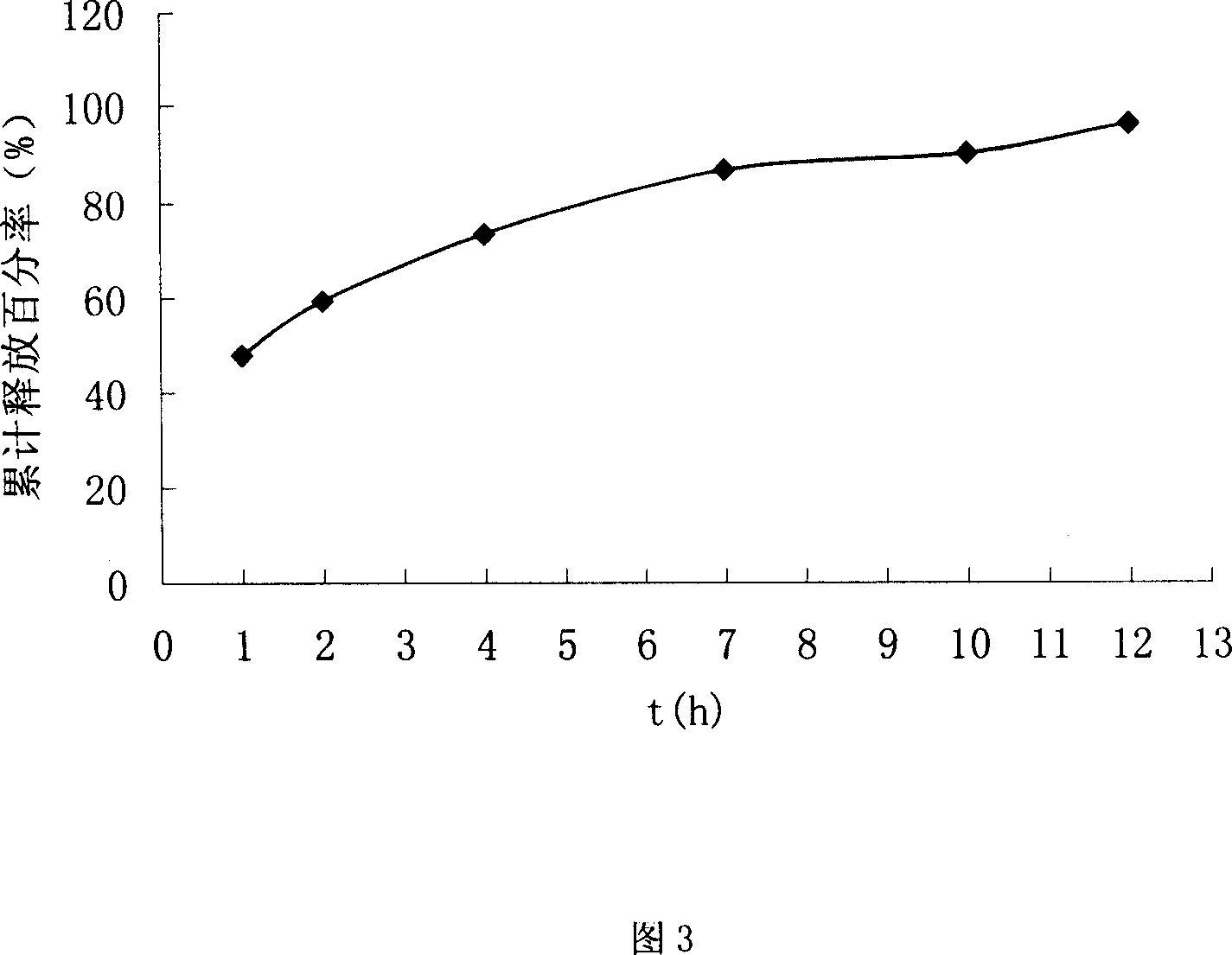
Chiral composition containing dextrothyroxine buprofenli and levomethadyl cysteliqin and its double slow-releasing tablet
A kind of technology of levocetirizine and dextro-ibuprofen, which is applied in the field of pharmaceutical compositions for treating colds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0082] Compound Dexibuprofen / Levocetirizine Dihydrochloride Double Layer Tablets (administered once every 12 hours, 2 tablets at a time)
[0083] Immediate Release Layer Prescription
60g
Levocetirizine dihydrochloride
1.25g
59.75g
40g
compressible starch
25g
Crospovidone
12g
8% povidone K30 in 95% ethanol solution
Appropriate amount
silica
1g
1g
Slow-release layer prescription
Dexibuprofen (or its pharmaceutically acceptable salt)
140g
Hydroxypropyl Methyl Cellulose K100m
12.5g
Hydroxypropyl Methyl Cellulose K4m
12.5g
45g
20g
compressible starch
18g
Food coloring (such as sunset yellow aluminum tint, etc.)
Appropriate amount
8% polyvinylpyrrolidone 70% ethanol solution
...
Embodiment 2
[0088] Compound Dexibuprofen / Levocetirizine Dihydrochloride Double Layer Tablets (administered once every 12 hours, 2 tablets at a time)
[0089] Immediate Release Layer Prescription
Dexibuprofen
70g
Levocetirizine dihydrochloride
1.25g
49.75g
40g
compressible starch
25g
12g
silica
1g
1g
Slow-release layer prescription
Dexibuprofen (or its pharmaceutically acceptable salt)
130g
Hydroxypropyl Methyl Cellulose K15m
25g
25g
lactose
30g
20g
compressible starch
18g
Food coloring (such as sunset yellow aluminum tint, etc.)
Appropriate amount
8% polyvinylpyrrolidone 80% ethanol solution
Appropriate amount
silica
1g
1g
...
Embodiment 3
[0094] Compound Dexibuprofen / Levocetirizine Dihydrochloride Double Layer Tablets (administered once every 12 hours, 2 tablets at a time)
[0095] Immediate Release Layer Prescription
Dexibuprofen
50g
Levocetirizine dihydrochloride
1.25g
lactose
69.75g
40g
compressible starch
25g
12g
95% ethanol
Appropriate amount
silica
1g
1g
Slow-release layer prescription
Dexibuprofen (or its pharmaceutically acceptable salt)
150g
Hydroxypropyl Methyl Cellulose K100m
20g
lactose
40g
20g
compressible starch
18g
Food coloring (such as sunset yellow aluminum tint, etc.)
Appropriate amount
8% polyvinylpyrrolidone 75% ethanol solution
Appropriate amount
silica
1g
PUM
| Property | Measurement | Unit |
|---|---|---|
| Hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com