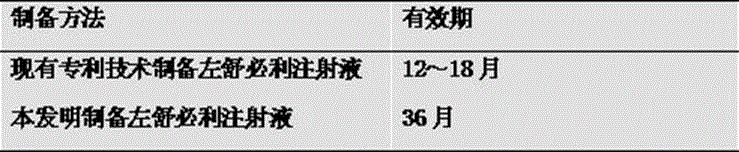
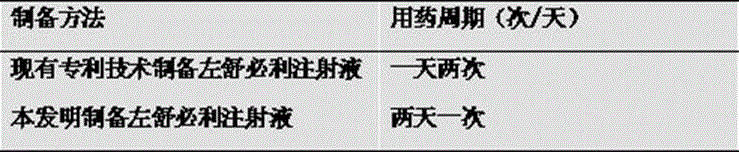
Levosulpiride injection and preparation method thereof
A technology of levosulpiride and injection, applied in the field of levosulpiride injection and its preparation, can solve the problems of poor patient compliance, short medication cycle, short validity period, etc., and achieve the effect of good stability and high encapsulation rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Take 19 g of gelatin, 4 g of polymethyl methacrylate, and 9 g of gum arabic, add 200 g of water for injection, stir for 30 minutes, let stand for 12 hours, filter with a vertically fused glass sand funnel to obtain a colloidal solution, and place it in an ultrasonic reactor; Take 50g of levosulpiride and 5g of D-tartaric acid, mix for 30 minutes, use a high-energy nano ball mill to pulverize into a nano-mixed powder of 150-200nm, add it to the colloid solution, select an ultrasonic frequency of 33kHz, react for 1 hour, add 20% formaldehyde solution, and continue the reaction For 30 minutes, add 600 g of water for injection, and place it in a spray freezer, select the spray freezing temperature -20°C, pressure 25 Pa, and spray to obtain levosulpiride nanocapsules; add 5 g of sodium chloride and 1000 g of water for injection, stir for 10 minutes, add Adjust the pH to 5.5 with glacial acetic acid, add 1 g of activated carbon and stir for 30 minutes, filter and decarbonize w...
specific Embodiment 2
[0033] Take 21g of gelatin, 6g of polymethyl methacrylate and 11g of gum arabic, add 200g of water for injection, stir for 30 minutes, let it stand for 12 hours, and filter it with a vertical melting glass sand funnel to obtain a colloidal solution, put it in an ultrasonic reactor; Sulpiride 50g, D-tartaric acid 5g, mixed for 30 minutes, crushed into 150-200nm nano-mixed powder with a high-energy nano ball mill, added to the colloid solution, selected ultrasonic frequency 33kHz, reacted for 1 hour, added 20% formaldehyde solution, and continued to react for 30 minutes , add 600g of water for injection, place in a spray freezer, select spray freezing temperature -20°C, pressure 25Pa, spray to obtain levosulpiride nanocapsules; add 5g of sodium chloride, 1000g of water for injection, stir for 10 minutes, add glacial acetic acid Adjust the pH to 6, add 1 g of activated carbon and stir for 30 minutes, filter and decarbonize with a 0.45 μm microporous membrane, pour the filtrate int...
specific Embodiment 3
[0035] Take 20kg of gelatin, 5kg of polymethyl methacrylate, and 10kg of gum arabic, add 200kg of water for injection, stir for 30 minutes, let stand for 12 hours, filter the colloidal solution with a vertical melting glass sand funnel, and put it in an ultrasonic reactor; Sulpiride 50kg, D-tartaric acid 5kg, mixed for 30 minutes, crushed into 150-200nm nano-mixed powder with a high-energy nano ball mill, added to the colloid solution, selected ultrasonic frequency 33kHz, reacted for 1 hour, added 20% formaldehyde solution, and continued to react for 30 minutes , add 600kg of water for injection, place in a spray freezer, select spray freezing temperature -20°C, pressure 25Pa, spray to obtain levosulpiride nanocapsules; add 5kg of sodium chloride, 1000kg of water for injection, stir for 10 minutes, add glacial acetic acid Adjust the pH to 5.8, add 1 kg of activated carbon and stir for 30 minutes, filter and decarbonize with a 0.45 μm microporous membrane, pour the filtrate into...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com