Isoquinoline triarylamine cyclic metal complex and its uses
A technology of isoquinolinyl triarylamine and ring metal, which is applied in the application field of polymer electroluminescent devices, can solve the problems of reduced luminous efficiency of electrophosphorescent devices, reduction of luminous efficiency and life of devices, aggregation of electrophosphorescent molecules, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
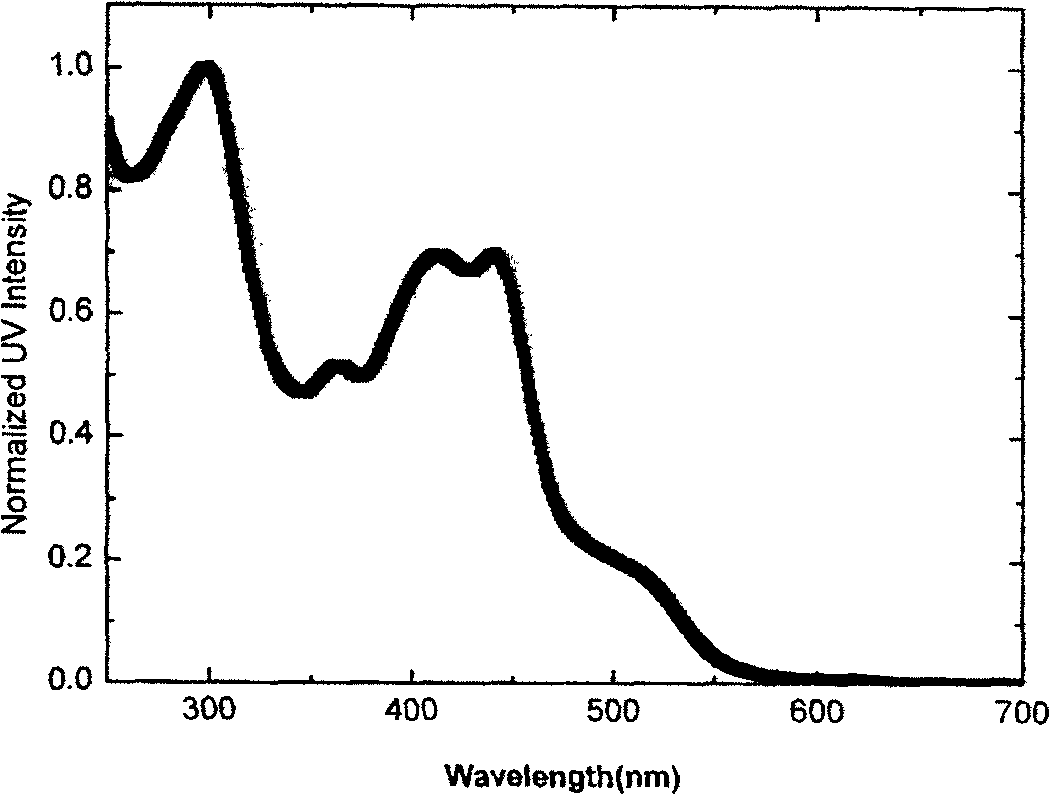
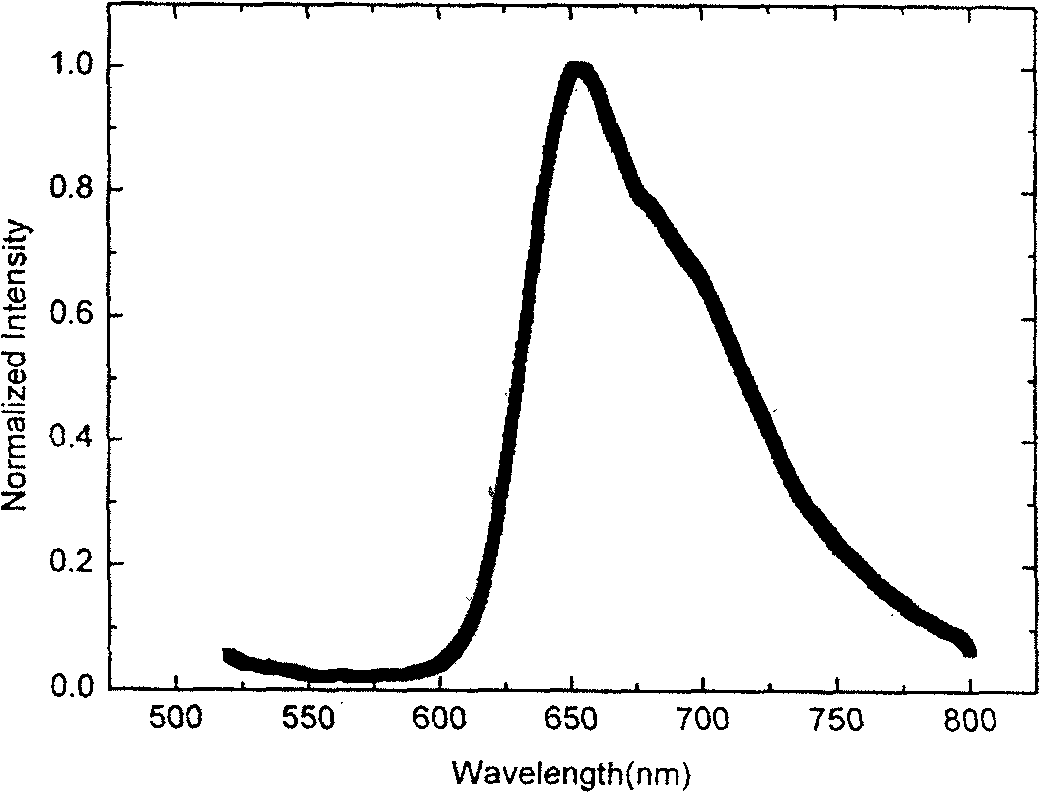
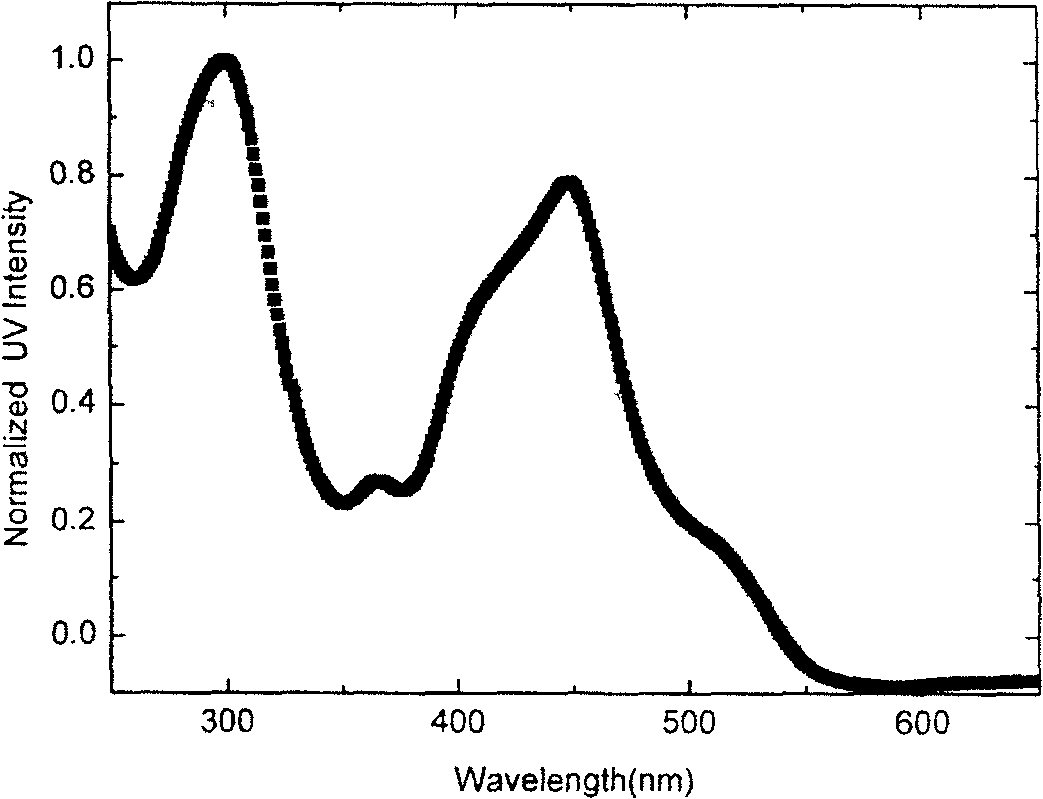
Image
Examples
Embodiment 1
[0084] Synthesis of 4-(1-isoquinolinyl)aniline
[0085]
[0086] 1. Synthesis of p-nitrobenzoyl chloride:
[0087] Add 30g (179mmol) p-nitrobenzoic acid to a 250mL single-neck bottle, install a condenser tube, quickly pour 76mL of thionyl chloride from the upper end of the condenser tube, install a drying tube, and reflux for 5 hours to obtain a pale yellow liquid, normal pressure The excess thionyl chloride was recovered by distillation and cooled to obtain 31.5 g of a pale yellow solid with a yield of 94.0%.
[0088] 2. Synthesis of N-(2-phenylethyl) p-nitrobenzamide:
[0089] In a 500mL three-necked flask, add 31.5g (169mmol) of p-nitrobenzoyl chloride, dissolve in 350mL of dichloromethane, add 22.2mL of β-phenethylamine dropwise under an ice-water bath, then add 26mL of triethylamine, the solution The turbidity became orange-red and transparent, and stirring was continued at room temperature for 26 hours. Pour the solution into a 1000mL beaker, add about 150mL of dic...
Embodiment 2
[0097] Synthesis of isoquinolinyl triarylamine
[0098]
[0099] R=hydrogen atom, tert-butyl, methyl, methoxy.
[0100] In a 50mL three-necked flask, 1.3g (5.9mmol) of 1-(4-aminophenyl)isoquinoline, 14.5mmol of substituted iodobenzene, 6.6g (47.8mmol) of anhydrous potassium carbonate, 1.5g of copper powder, 0.4 g dibenzo-18-crown-6-ether and 25 mL of o-dichlorobenzene were heated and refluxed for 24 hours under nitrogen protection, cooled, the reaction mixture was suction filtered, and o-dichlorobenzene was recovered by distillation under reduced pressure. Using ~300 mesh silica gel as the stationary phase and dichloromethane as the eluent, the product was separated by column chromatography to obtain a yellow solid product.
[0101] N,N-Diphenyl-4-(1'-isoquinolinyl)aniline (DPAPiq), yield 77.6%, m.p.142-144°C. 1 H NMR (400MHz, CDCl 3 )δppm: 8.60~8.59(d, 1H), 8.26~8.24(d, 1H), 7.90~7.88(d, 1H), 7.73~7.69(t, 1H), 7.64~7.56(m, 4H), 7.32~ 7.19 (m, 10H), 7.09-7.05 (t, 2H). ...
Embodiment 3
[0106] Synthesis of Isoquinolinyl Triarylamine Ring Metal Iridium Complex Electrophosphorescent Materials
[0107]
[0108] R=hydrogen atom, tert-butyl, methyl, methoxy.
[0109] In a 50mL three-necked flask, add 1.62mmol of the compound of Example 2, 15mL of ethylene glycol monoethyl ether and 5mL of water, stir magnetically, heat through nitrogen for deoxygenation for 30 minutes, and after cooling, quickly add 142mg (0.40mmol) of IrCl 3 ·3H 2 O, under the protection of nitrogen, the reaction was heated to reflux for 25 hours, cooled, and a red precipitate was precipitated. Suction filtration, the precipitate was washed with 95% ethanol (5mL×2), acetone (5mL×2) and n-hexane (5mL×2) successively, and dried in vacuum to obtain a red dimer solid of chloride-bridged metal iridium .
[0110] 0.15 mmol dimer solid, 40.2 mg (0.40 mmol) acetylacetone, 20.3 mg Na 2 CO 3 and 15mL of ethylene glycol monoethyl ether, added to a 50mL three-necked flask, under nitrogen protection, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| emission peak | aaaaa | aaaaa |
| luminance | aaaaa | aaaaa |
| emission peak | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com