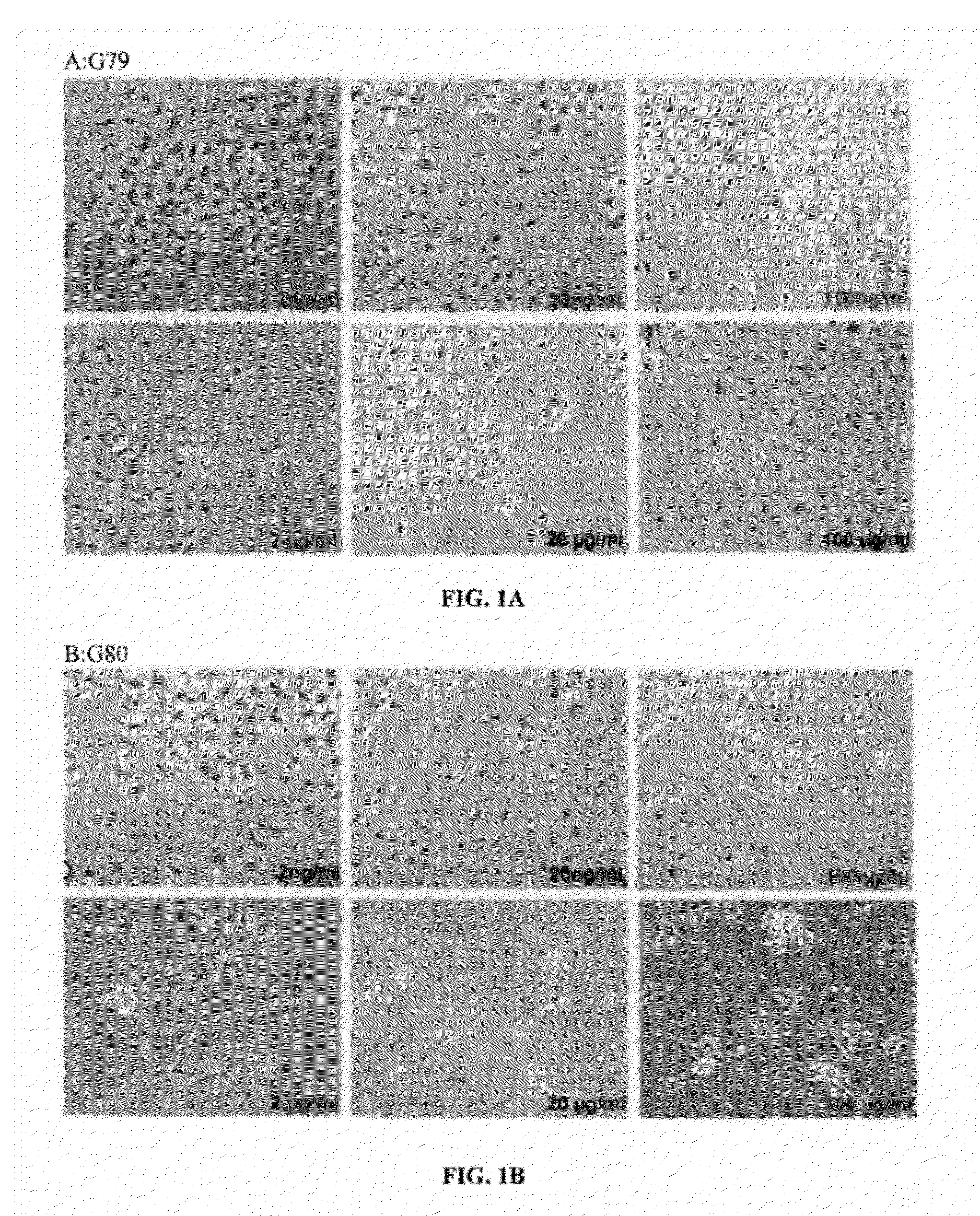
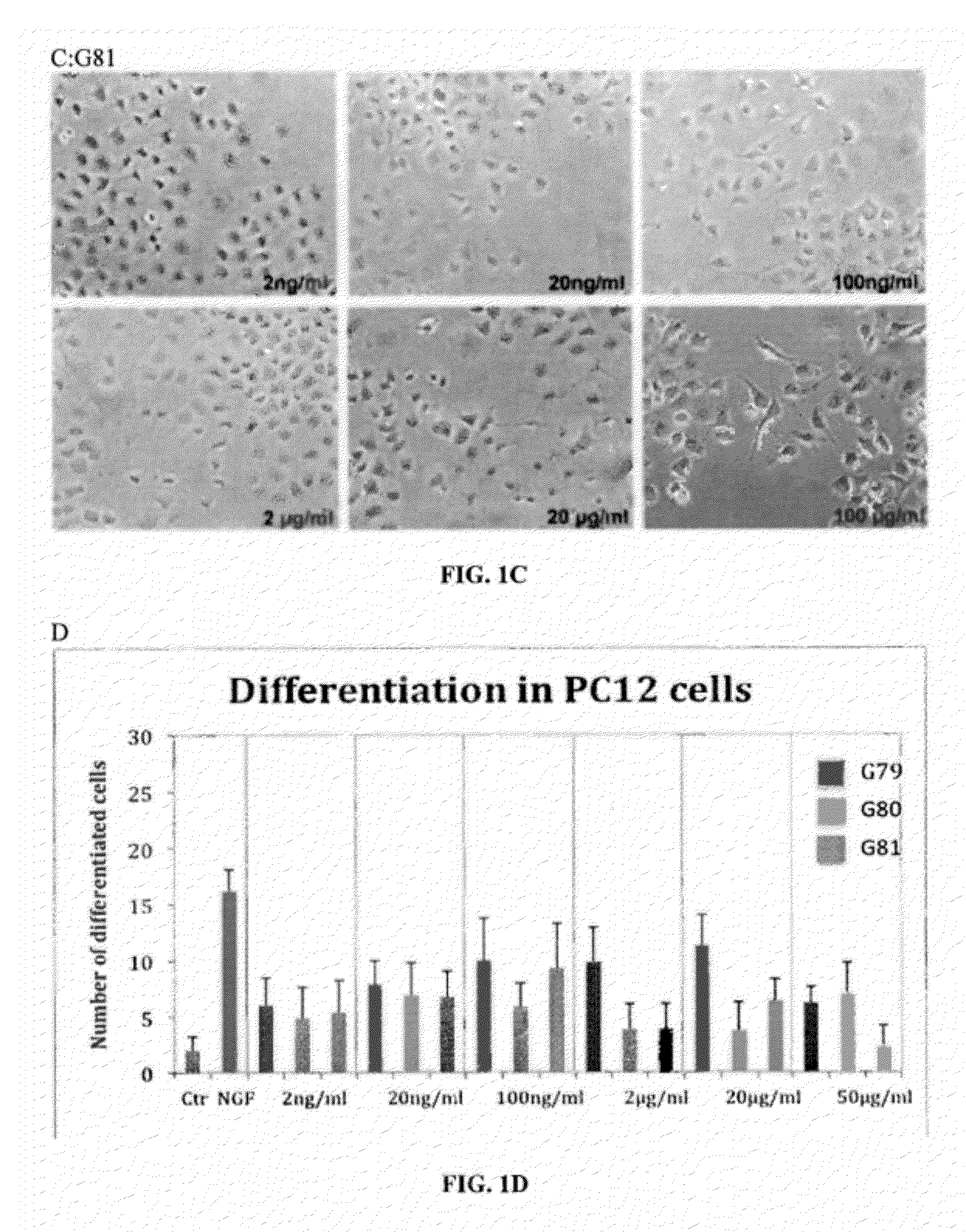
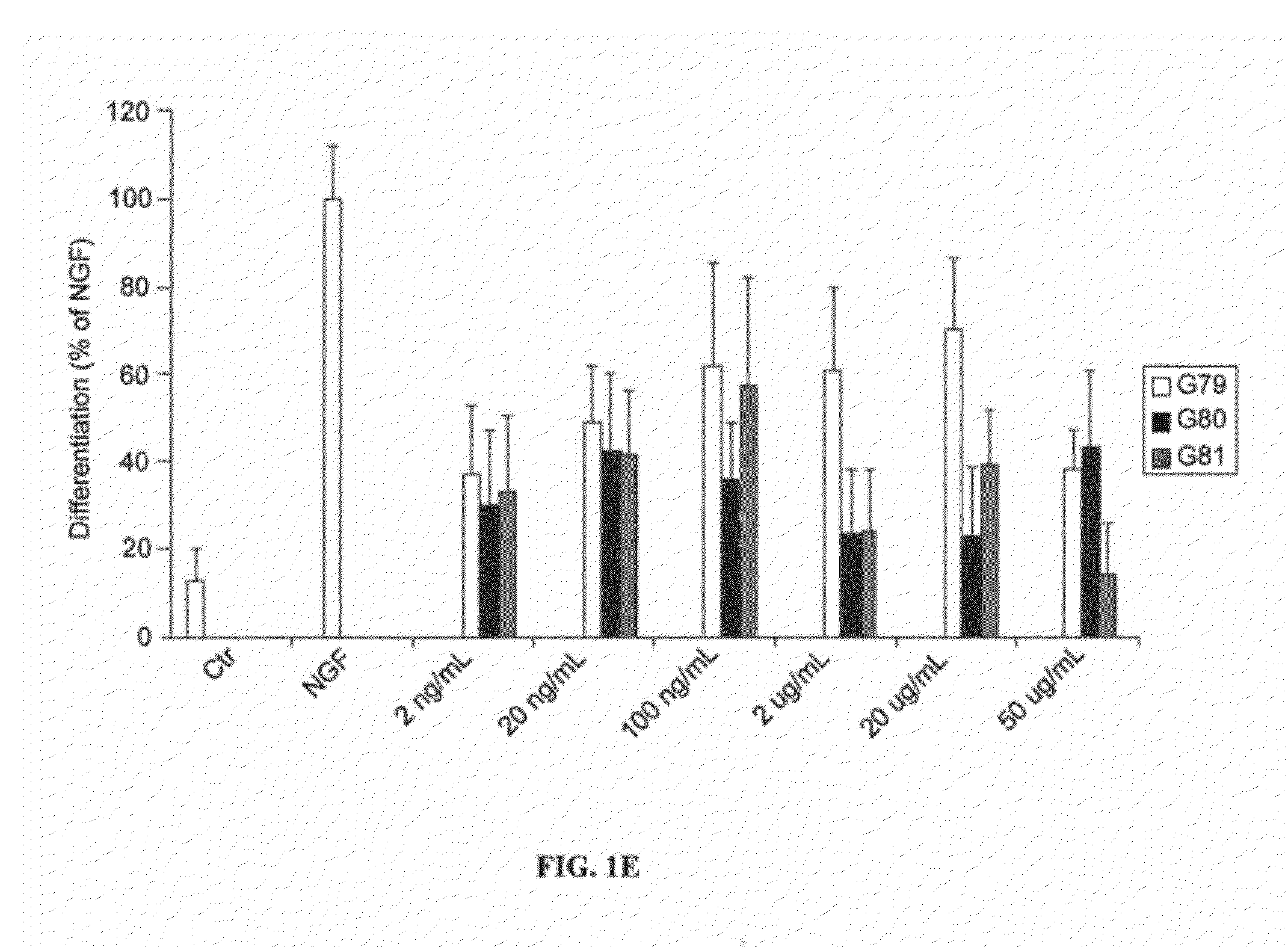
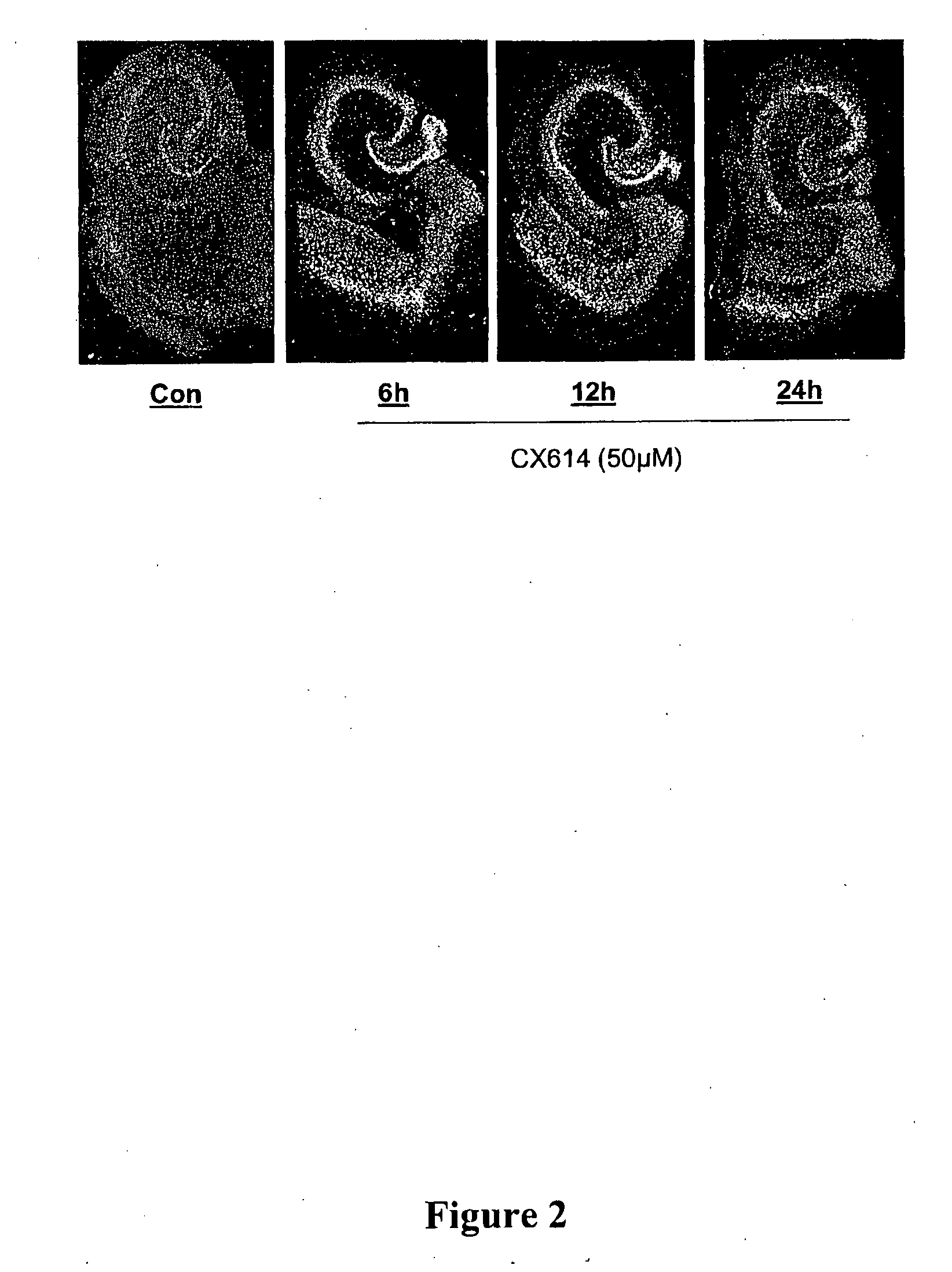
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
57 results about "Neurotrophic Proteins" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Methods for therapy of neurodegenerative disease of the brain
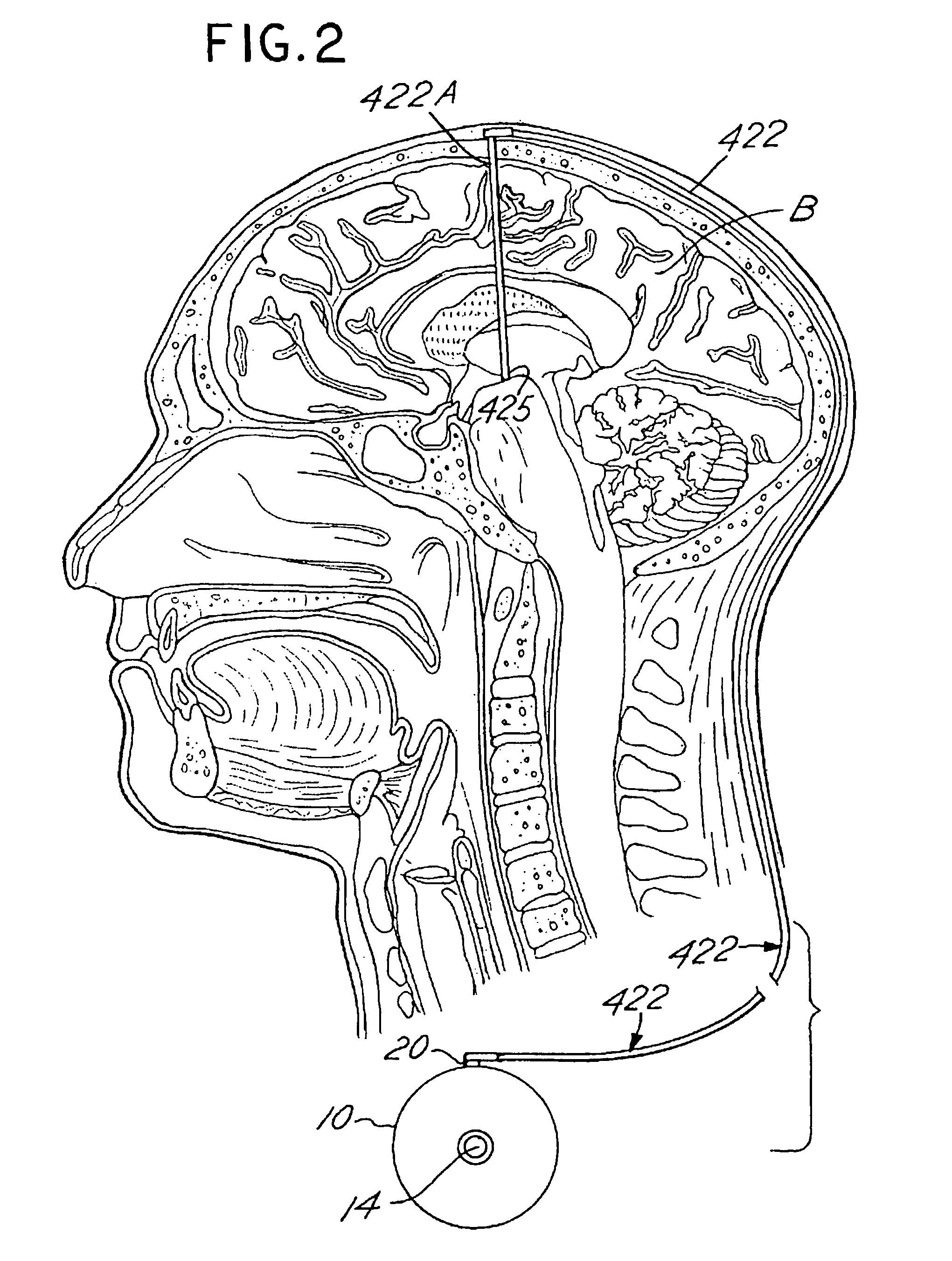
A specific clinical protocol for use toward therapy of defective, diseased and damaged cholinergic neurons in the mammalian brain, of particular usefulness for treatment of neurodegenerative conditions such as Alzheimer's disease. The protocol is practiced by delivering a definite concentration of recombinant neurotrophin into, or within close proximity of, identified defective, diseased or damaged brain cells. Using a viral vector, the concentration of neurotrophin delivered as part of a neurotrophic composition varies from 10<10 >to 10<15 >neurotrophin encoding viral particles / ml of composition fluid. Each delivery site receives from 2.5 mul to 25 mul of neurotrophic composition, delivered slowly, as in over a period of time ranging upwards of 10 minutes / delivery site. Each delivery site is at, or within 500 mum of, a targeted cell, and no more than about 10 mm from another delivery site. Stable in situ neurotrophin expression can be achieved for 12 months, or longer.
Owner:RGT UNIV OF CALIFORNIA
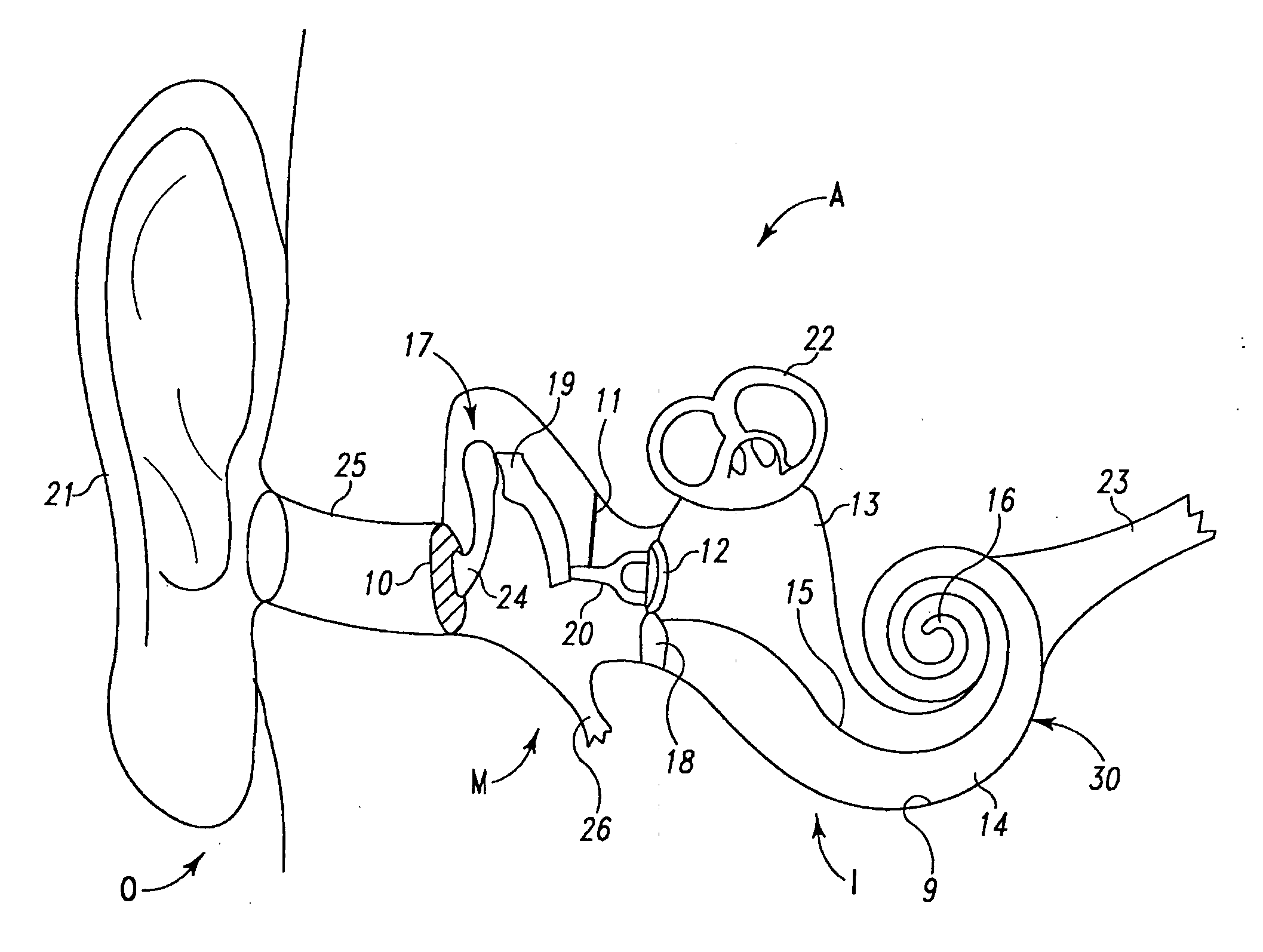
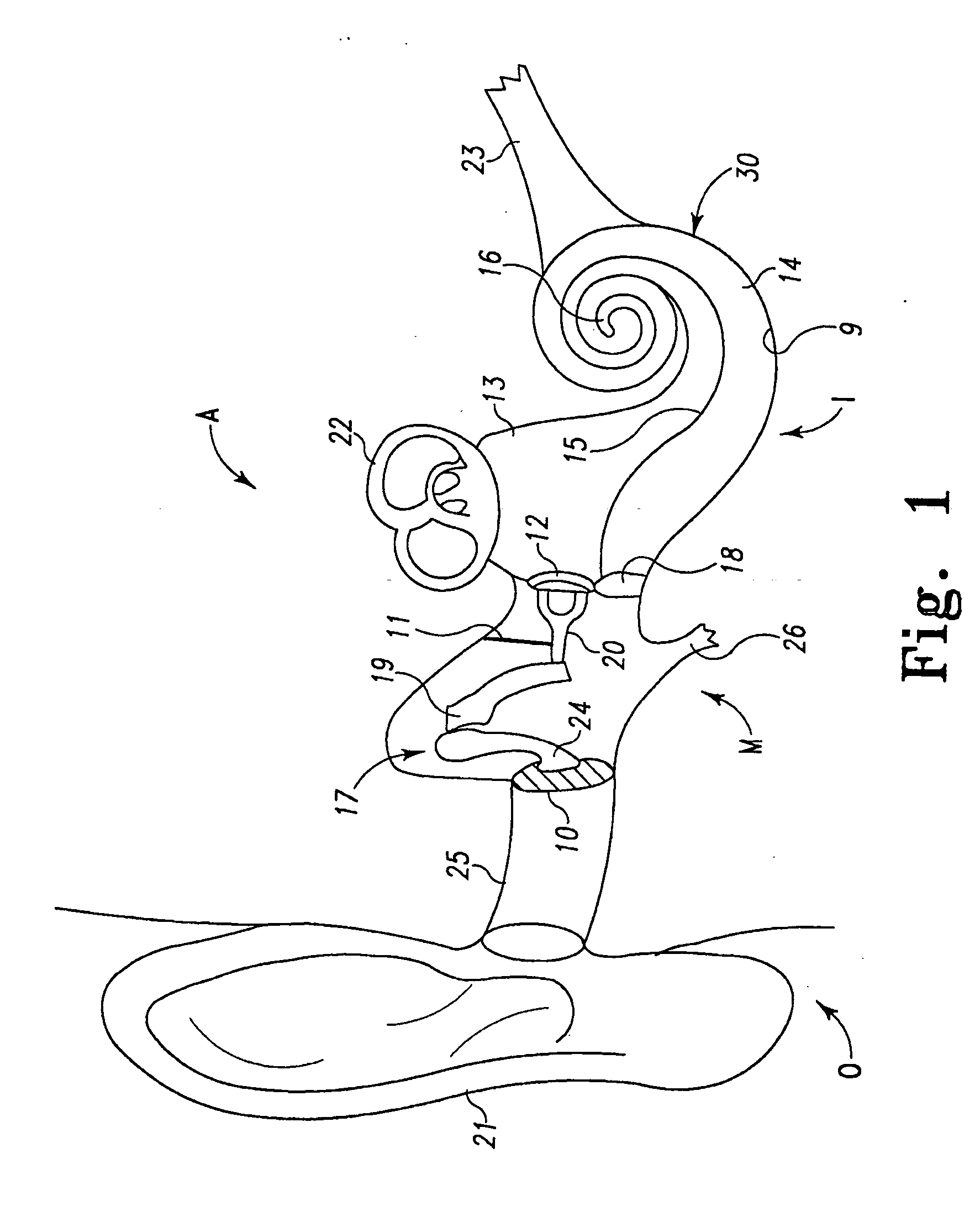
Extra-cochlear implanted hearing aid device
ActiveUS20070005117A1Promote growthEasily and efficiently transmitHead electrodesHearing aidTunica intima
An extra-cochlear hearing aid implant is characterized by a pad having a plurality of electrode prongs that extend therefrom and which are adapted to provide an electrical stimulus to hearing cells within the cochlea. The electrode pad is adapted to be placed onto endosteum overlying the cochlea in an “extended soft surgery” technique. The prongs are configured to pierce the endosteum and extend into the cochlea. In one form, the extra-cochlear hearing aid implant also includes hollow tubules that extend from the pad and which are adapted to supply and withdraw neurotrophic proteins and other materials in a fluid into and from the cochlea, and also to provide electrical stimulus to the hearing cells.
Owner:DOMESTIC ASSET LLP
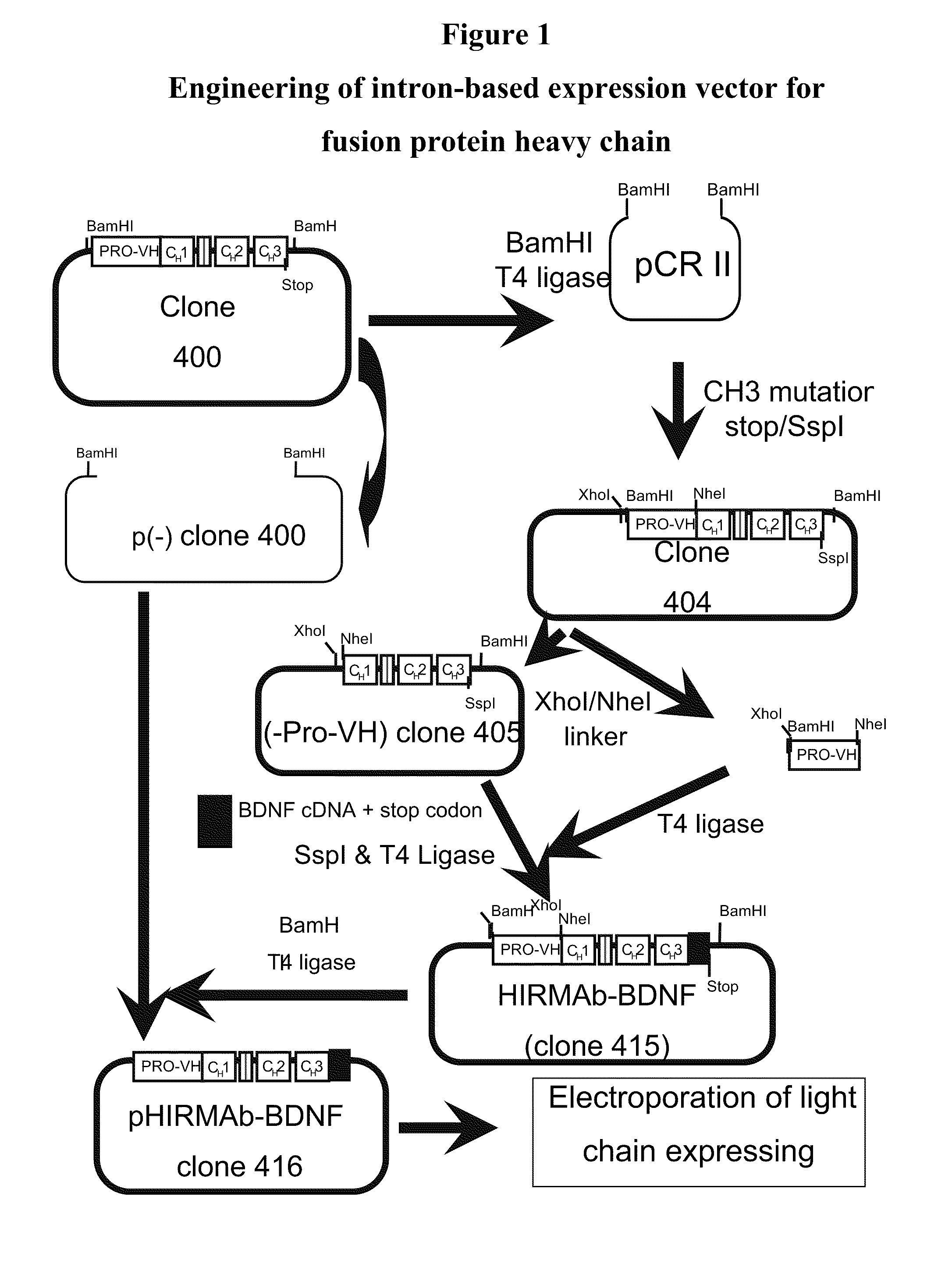
Fusion proteins for delivery of erythropoietin to the CNS
ActiveUS8124095B2Effective treatmentNervous disorderPeptide/protein ingredientsNeurotrophic ProteinsMediated transport
The invention provides compositions, methods, and kits for increasing transport of a neurotrophin (e.g., erythropoietin (EPO)) across the blood brain barrier while allowing its activity to remain substantially intact. The neurotrophin (e.g., EPO) is transported across the blood brain barrier via one or more endogenous receptor-mediated transport systems.
Owner:ARMAGEN TECH +1
Method(s) of stabilizing and potentiating the actions and administration of brain-derived neurotrophic factor (BDNF)
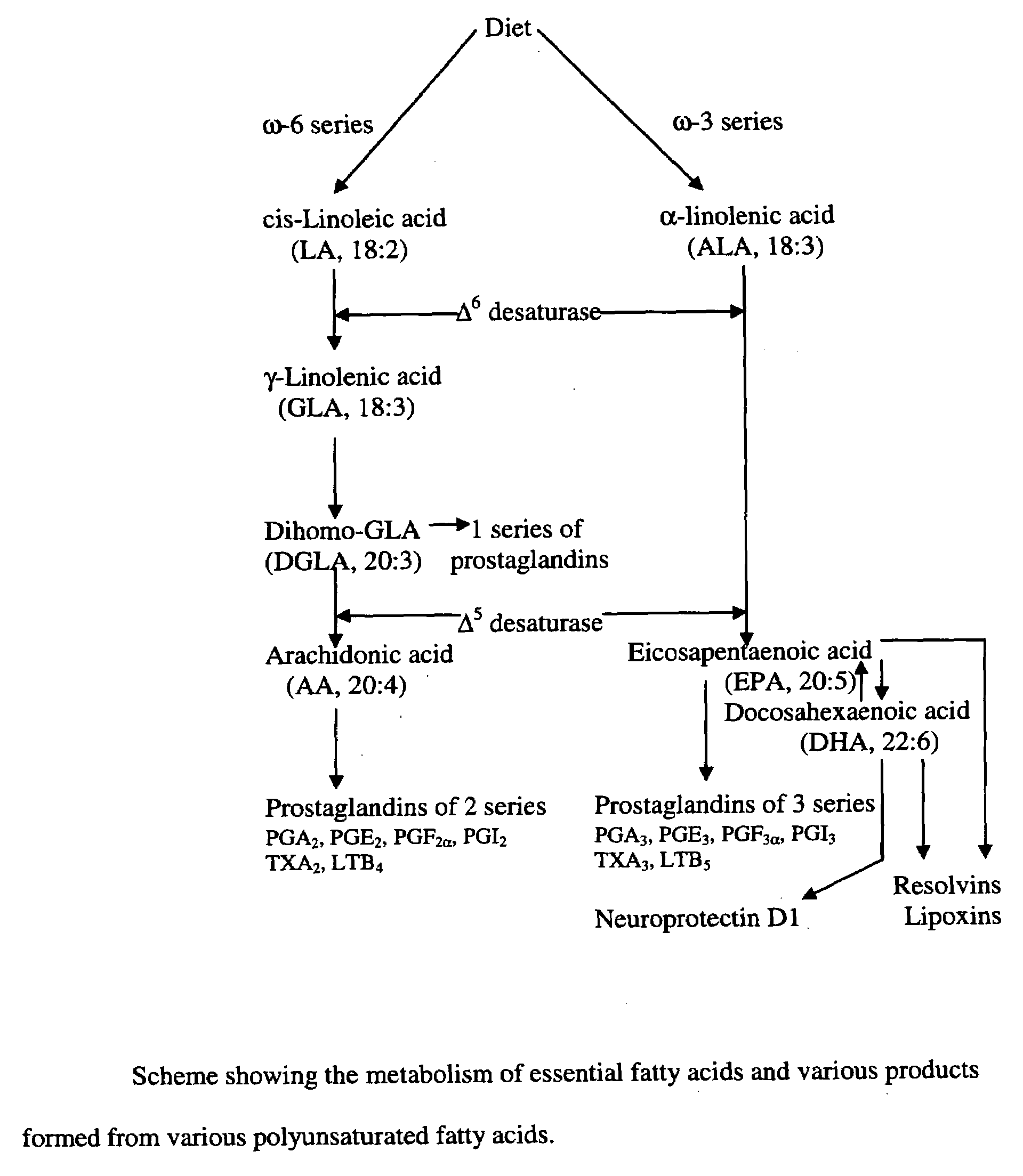
A method of stabilizing and potentiating actions and administration of neurotrophins such as brain-derived neurotrophic factor (BDNF), neurotrophin-3, neurotrophin-4, and nerve growth factor and their receptors by using in coupling conjugation with polyunsaturated fatty acids (PUFAs) in the prevention and / or treatment of obesity, type 2 diabetes mellitus, metabolic syndrome X, Alzheimer's disease, depression, Parkinson's disease, and schizophrenia is described. The invention is directed to the efficacious use of various neurotrophins by using them in combination with polyunsaturated fatty acids chosen from linoleic acid, gamma-linolenic acid, dihomo-gamma-linolenic acid, arachidonic acid, alpha-linolenic acid, eicosapentaenoic acid, docosahexaenoic acid, cis-parinaric acid, docosapentaenoic acid and conjugated linoleic acid in predetermined quantities. The invention also provides methods of efficiently delivering neurotrophins to the desired areas of the brain by complexing or conjugating with PUFAs, so that they are able to cross blood brain barrier efficiently and reach the desired regions of the brain in adequate amounts.
Owner:DAS UNDURTI N
Recombinant polypeptide useful for neurotrophin receptor mediated gene delivery and as neurotrophin agonist
InactiveUS7205387B2High activityPeptide/protein ingredientsFermentationGene deliveryNeurotrophin Receptor p75
The invention provides a novel recombinant polypeptide that comprises a nucleic acid binding element and a hairpin motif that selectively binds to a neurotrophin receptor. The recombinant polypeptide may be used for neurotrophin receptor mediated delivery of nucleic acid, including therapeutic DNA, bound to the recombinant polypeptide. In one embodiment, the hairpin motif is a hairpin motif of a neurotrophin, such as nerve growth factor, brain derived neurotrophic factor, neurotrophin 3 and neurotrophin 4 / 5. The hairpin motif is also a neurotrophin agonist and therefore may be used to treat any disorder responsive to neurotrophin treatment, such as neurological disorders and tumour. In one embodiment the agonist comprises a hairpin motif that selectively binds to a neurotrophin receptor and a positively charged binding domain which is believed to enhance receptor binding by binding to negatively charged cell membrane.
Owner:AGENCY FOR SCI TECH & RES
Methods for therapy of neurodegenerative disease of the brain
InactiveUS6815431B2Improve the level ofMinimal toxicityBiocidePeptide/protein ingredientsMammalMammalian brain
Owner:RGT UNIV OF CALIFORNIA
Active or passive immunization against proapoptotic neurotrophins for the treatment and/or prevention of neurodegenerative diseases
InactiveUS20060275290A1Potent apoptotic activityReduce neuronal or glial cell apoptosisNervous disorderPeptide/protein ingredientsAdjuvantNeuro-degenerative disease
The present invention relates to novel methods for combating cell degeneration or dysfunction resulting from neuroinflammatory conditions. The invention especially relates to the use, in the preparation of a medicament for the treatment of neurodegenerative disease associated with neuroinflammation, of an immunogenic compound which is capable of inducing an immune response against a proapoptotic neurotrophin, or an effective amount of a hapten combined with appropriate carriers and / or adjuvants to render the resulting combination capable of inducing an immune response against a proapoptotic neurotrophin. Also disclosed are compositions for the active or passive immunization against neuronal or glial cell apoptosis caused by neuroinflammation as well as methods and means useful for said active or passive immunization.
Owner:INST PASTEUR +1
Pantropic neurotrophic factors
Pantropic neurotrophic factors which have multiple neurotrophic specificities are provided. The pantropic neurotrophic factors of the present invention are useful in the treatment of neuronal disorders. Nucleic acids and expression vectors encoding the pantropic neurotrophins are also provided.
Owner:GENENTECH INC
Methods of modulating neurotrophin-mediated activity
InactiveUS20090082368A1Modulate activityBiocideNervous disorderNeurotrophic ProteinsNerve growth factor
Disclosed are compositions which modulate the interaction with nerve growth factor and precursors thereof with neurotrophic receptors. Also disclosed are methods of using the compositions of the invention, including methods of administration.
Owner:PAINCEPTOR PHARMA CORP
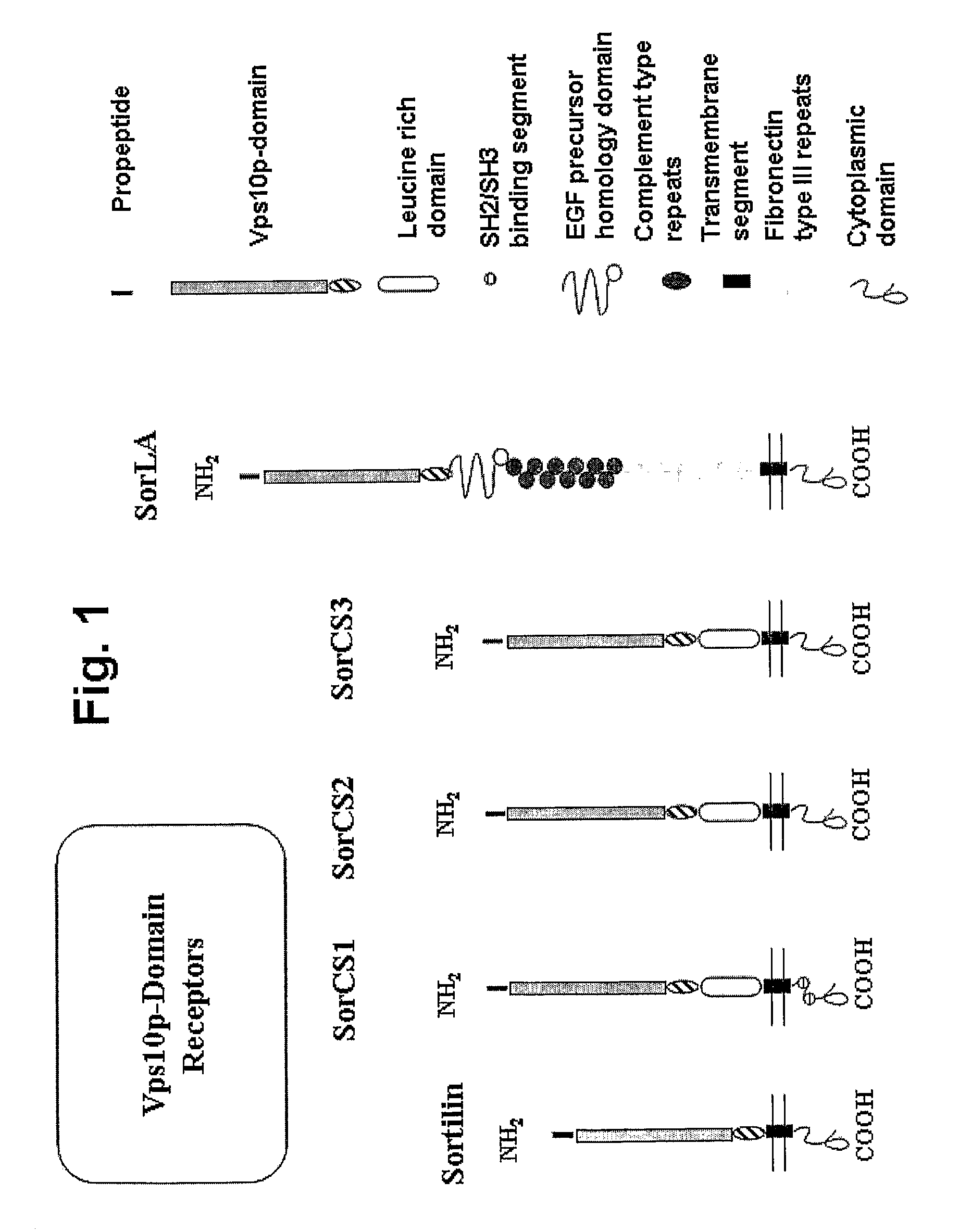
Modulation of activity of proneurotrophins
The present invention provides agents for inhibiting binding of a pro-neurotrophin to a Vps10p-domain receptor, in particular the binding of a pro-NGF or a pro-BDNF to a Sortilin receptor. The invention thus provides agents for the manufacture of a medicament, for treating and / or preventing disease or disorders such as but not limited to neurological, neuropsychiatric and ocular diseases, disorders, and degeneration as well as obesity, diabetes, pain and / or nociception in an individual.
Owner:H LUNDBECK AS
Methods for therapy of neurodegenerative disease of the brain
A specific clinical protocol for use toward therapy of defective, diseased and damaged cholinergic neurons in the mammalian brain, of particular usefulness for treatment of neurodegenerative conditions such as Alzheimer's disease. The protocol is practiced by delivering a definite concentration of recombinant neurotrophin into, or within close proximity of, identified defective, diseased or damaged brain cells. Using a viral vector, the concentration of neurotrophin delivered as part of a neurotrophic composition varies from 1010 to 1015 neurotrophin encoding viral particles / ml of composition fluid. Each delivery site receives from 2.5 μl to 25 μl of neurotrophic composition, delivered slowly, as in over a period of time ranging upwards of 10 minutes / delivery site. Each delivery site is at, or within 500 μm of, a targeted cell, and no more than about 10 mm from another delivery site. Stable in situ neurotrophin expression can be achieved for 12 months, or longer.
Owner:RGT UNIV OF CALIFORNIA
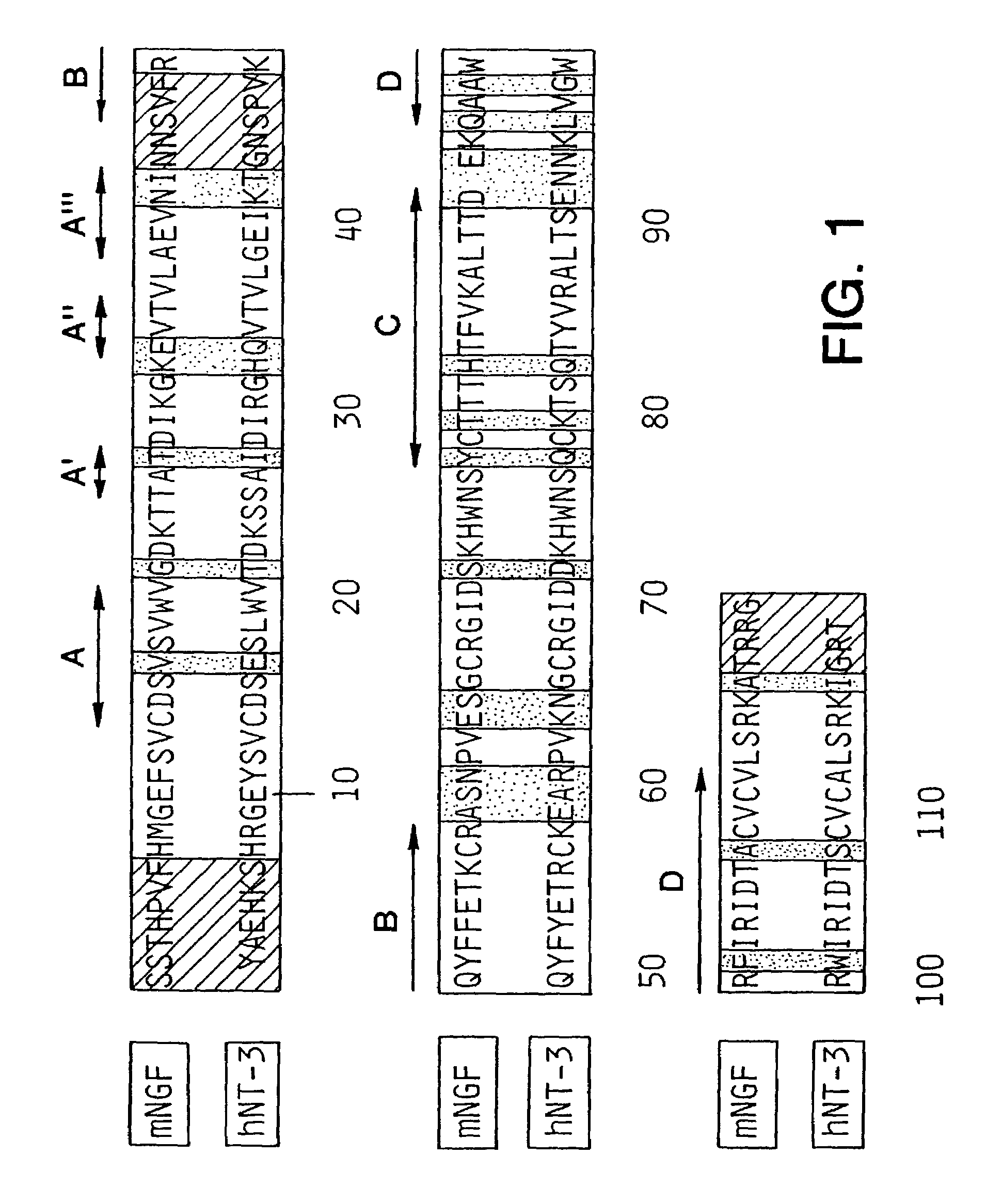
Neurotrophin-derived peptide sequences
The present invention relates to peptide sequences capable of stimulating neuronal cell differentiation, neural cell survival and neuronal plasticity associated with memory and learning. The peptide sequences of the invention are derived from the proteins belonging to neurotrophic factors, such as NGF, NT3, NT4 / 5 and BDNF. The invention also relates to pharmaceutical compositions comprising said peptide fragments and uses thereof for treatment of a disease or condition wherein the effects of stimulating neuronal cell differentiation, neuronal cell survival, stimulating neural plasticity associated with learning and memory are beneficial for treatment.
Owner:COPENHAGEN UNIV TECHTRANS UNIT
Regulation of neurotrophins
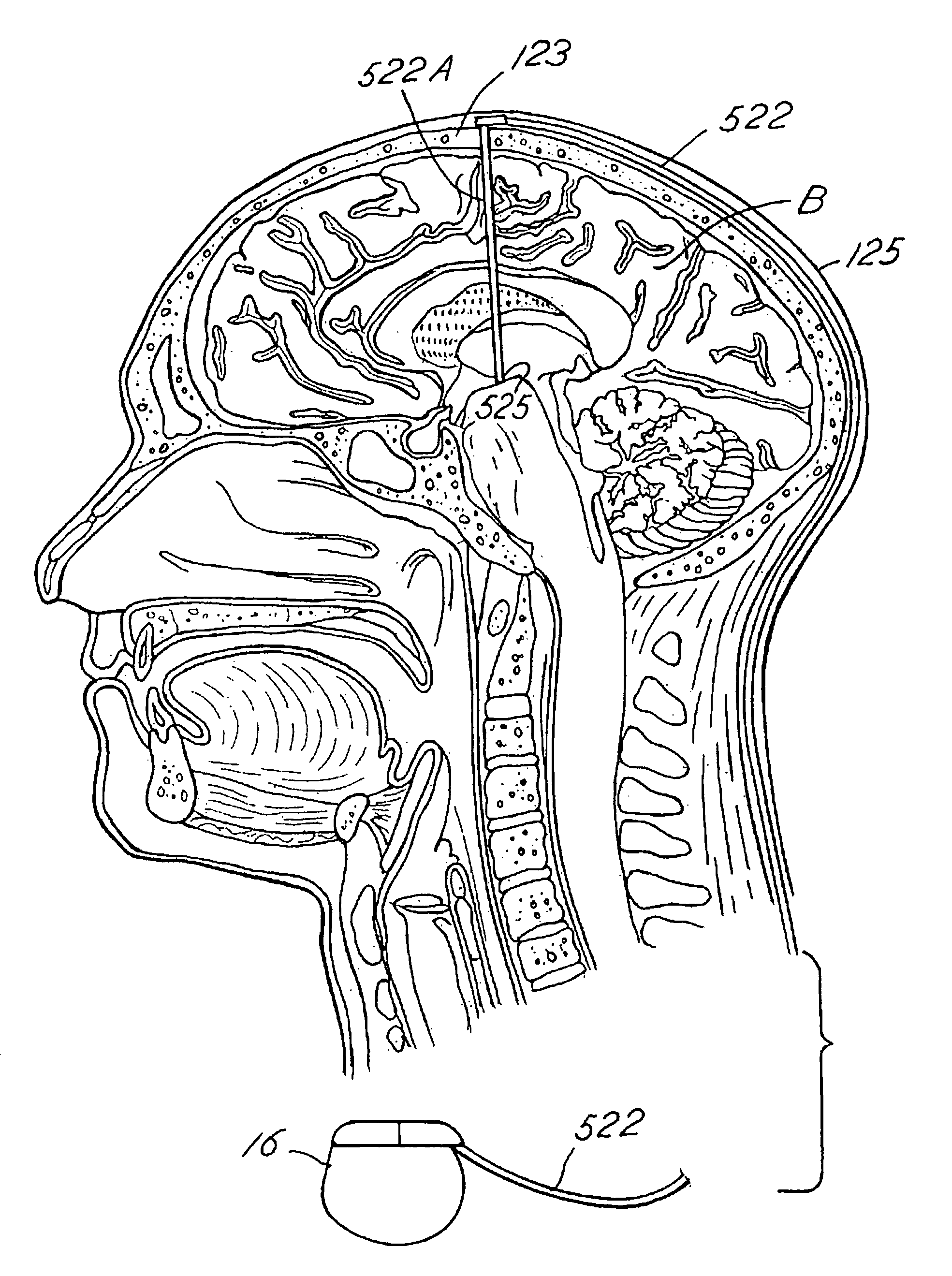
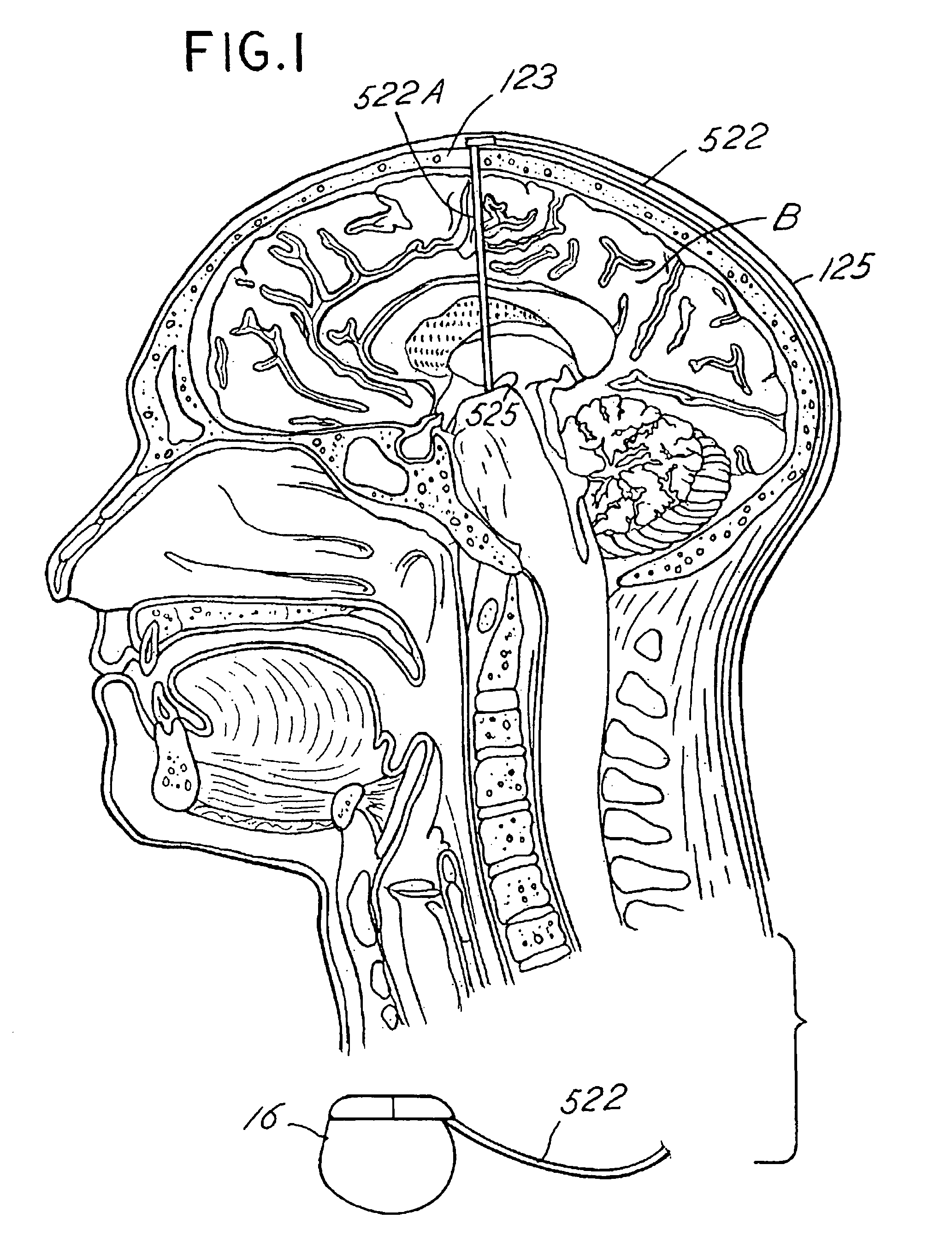
Disclosed are methods for regulating neurotrophin levels within a human body. The invention utilizes an implantable signal generator to deliver stimulation to neural tissue elements. Alternatively, an implantable pump may be utilized to delivery one or more drugs. The implanted device delivers treatment therapy to the neural tissue to thereby alter the level of neurotrophic factors such as BDNF expressed by the influenced neural tissue. A sensor may be used to detect various symptoms of a nervous system disorder. A microprocessor algorithm may then analyze the output from the sensor to regulate the treatment therapy delivered to the body. The invention describes a novel method to regulate the intrinsic levels of neurotrophins and may be used to treat patients with neurological and cognitive disorders.
Owner:FUNCTIONAL NEUROMODULATION
Antibodies that mimic actions of neurotrophins
InactiveUS7459156B2Inhibit bindingPromote activationCell receptors/surface-antigens/surface-determinantsPeptide/protein ingredientsImmunoglobulin IgETrk receptor
The use and production of immunoglobulins which activate trk receptors and imitate effects of neurotrophins are provided. Immunoglobulins which block trk receptor activation and methods of use are also provided.
Owner:RGT UNIV OF CALIFORNIA
Rescue of Photoreceptors by Intravitreal Administration of an Expression Vector Encoding a Therapeutic Protein
InactiveUS20090202505A1Increase amplitudeOrganic active ingredientsBiocideDiseaseTherapeutic protein
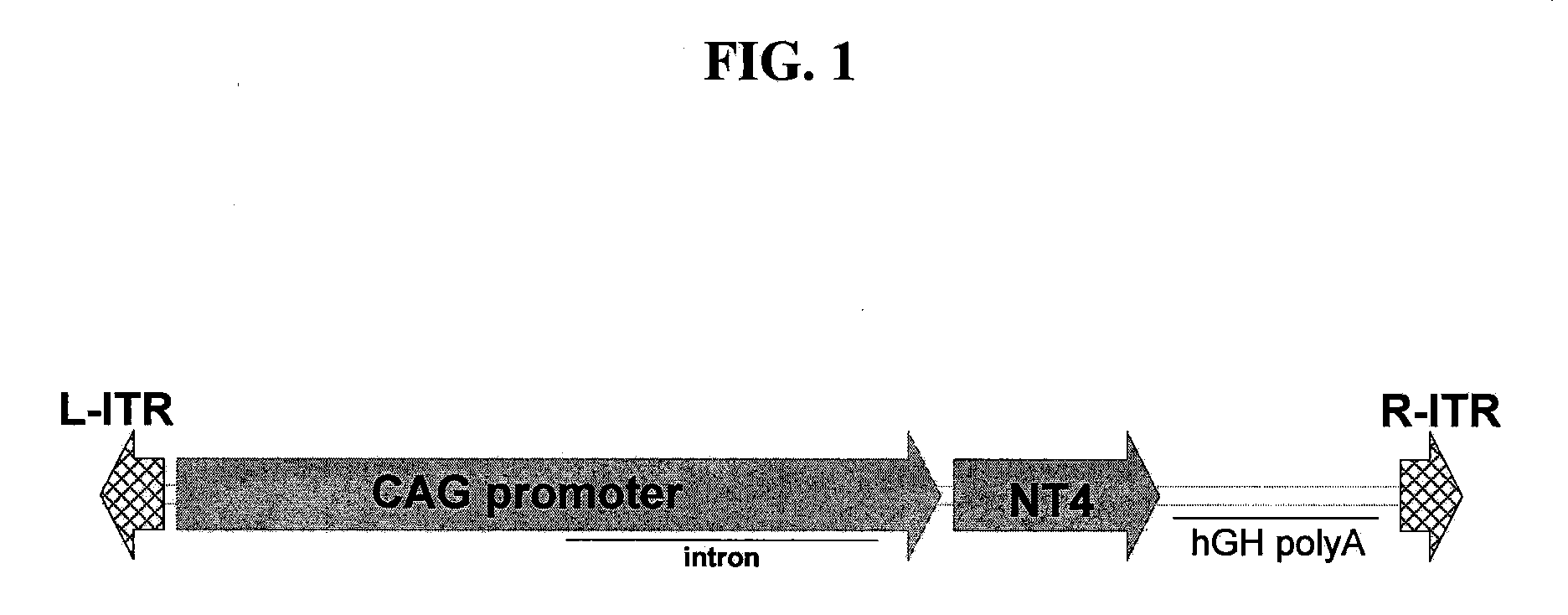
The invention provides methods for treating ocular diseases using a recombinant vehicle to express a protein useful in the treatment of ocular disease, with particular preference for use of neurotrophin-4 (NT4) for targeting subpopulations of cells in the retina. A genetically engineered gene transfer vector containing sequences encoding a growth factor such as neurotrophin-4 (NT4) is used to transduce cells of the retinal ganglion cell (RGC) layer, in situ, via administration of the vector intravitreally. Accordingly, methods are disclosed for treating subjects in need thereof by therapeutic protein delivery via a recombinant expression vector, including rescue of photoreceptors by targeting the RGC layer subpopulation of retinal cells.
Owner:CEREGENE
Method for screening molecules that exert a neurotrophic effect through activation of neurotrophin receptors
InactiveUS7169568B2Microbiological testing/measurementBiological testingNeurotrophin Receptor p75Phosphorylation
The present invention is directed to a method for screening and identifying molecules that transactivate a neurotrophin receptor and mediate neuronal cell survival in the absence of neurotrophins which uses one or a combination of three different assays. The assays involve detecting the phosphorylation of a neurotrophin receptor, detecting the phosphorylation of phosphotidylinositol 3′-kinase or Akt enzyme, and assessing neuronal cell survival in the absence of neurotrophins.
Owner:NEW YORK UNIV
Method for Preventing and Treating Diabetes Using Neurturin
InactiveUS20080241106A1Reduce the numberReduce in quantityBiocidePeptide/protein ingredientsDiseaseDiabetes mellitus
Owner:DEVELOGEN AKTIENGES
Trophic Factor Combinations for Nervous System Treatment
The present invention relates to a composition including an effective amount of at least one of an antimicrobial peptide and a substance having an antimicrobial peptide effect and an effective amount of a neurotrophin. The composition can also include an effective amount of at least one of a growth factor and a neuropeptide. The present invention also relates a method of treating an injury to a nervous system of an animal that includes the steps of identifying the injury to the nervous system and applying to the injury an effective amount of at least one of antimicrobial peptide and a substance having an antimicrobial peptide effect. The method can also include applying an effective amount of one or more trophic factors selected from the group consisting of a growth factor, a neurotrophin, and a neuropeptide to the injury.
Owner:WISCONSIN ALUMNI RES FOUND
Intranasal administration of agents with pro-inflammatory activity for the therapy of neurological disorders
ActiveUS20140290647A1Stimulate endogenous productionPromote productionOrganic active ingredientsBiocideSerotoninNervous system
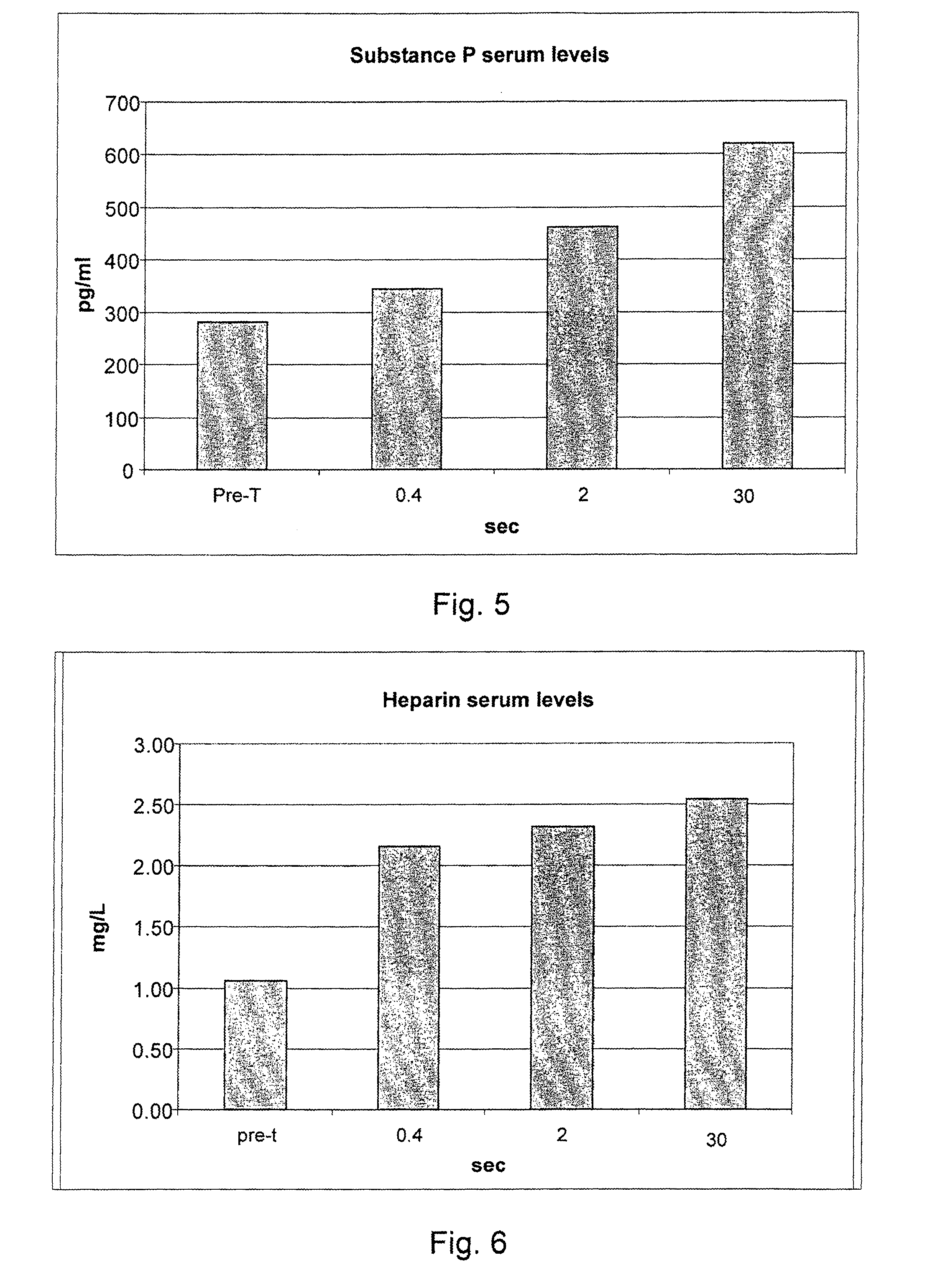
The invention concerns a product consisting of distilled water or of an aqueous solution hypotonic or substantially isotonic with respect to blood plasma or, less preferably, hypertonic with respect to blood plasma, to be administered on the nasal mucosa according to a specific operation mode in order to cause an inflammation of an irritative kind, for use in a treatment of stimulation of the endogenous production of inflammatory mediators, in the therapy and prevention of neurological disorders, in particular of degenerative disorders of the central and peripheral nervous system. The inflammatory mediators the endogenous production of which is stimulated comprise NGF, neurotrophin-3, neurotrophin-4, serotonin, substance P, heparin, ECF-A.
Owner:SALVINELLI BEATRICE
Modulation of activity of proneurotrophins
The present invention provides agents for inhibiting binding of a pro-neurotrophin to a Vps1 Op-domain receptor, in particular the binding of a pro-NGF or a pro-BDNF to a Sortilin receptor. The invention thus provides agents for the manufacture of a medicament, for treating and / or preventing disease or disorders such as but not limited to neurological, neuropsychiatric and ocular diseases, disorders, and degeneration as well as obesity, diabetes, pain and / or nociception in an individual.
Owner:H LUNDBECK AS
Pantropic neurotrophic factors
Pantropic neurotrophic factors which have multiple neurotrophic specificities are provided. The pantropic neurotrophic factors of the present invention are useful in the treatment of neuronal disorders. Nucleic acids and expression vectors encoding the pantropic neurotrophins are also provided.
Owner:GENENTECH INC
High affinity ligand for p75 neurotrophin receptor
InactiveUS7507799B2Antibacterial agentsOrganic active ingredientsNeurotrophin Receptor p75ADAMTS Proteins
The present invention provides an isolated protein comprising a pro-domain of a proneurotrophin, methods for producing the protein, and pharmaceutical compositions containing the isolated protein. The invention also provides a nucleic acid molecule which encodes the protein and a vector containing the nucleic acid molecule. The present invention further provides a method for cleaving a proneurotrophin protein to a mature neurotrophin. In addition, the invention relates to methods for inducing apoptosis in a cell of a mammal expressing p75 surface receptors or p75 and trk receptors. The methods include causing the p75 receptor to bind a pharmaceutical composition containing a pro-domain of a proneurotrophin or administering to the mammal an effective amount of a cleavage-resistant proneurotrophin and an inhibitor of trk activation. The invention also relates to a method for inhibiting apoptosis of a cell in a mammal by administering an effective amount of a molecule which inhibits binding of a proneurotrophin to a p75 receptor. Also provided, are kits and methods for screening a human for a condition associated with undesired apoptosis.
Owner:CORNELL RES FOUNDATION INC
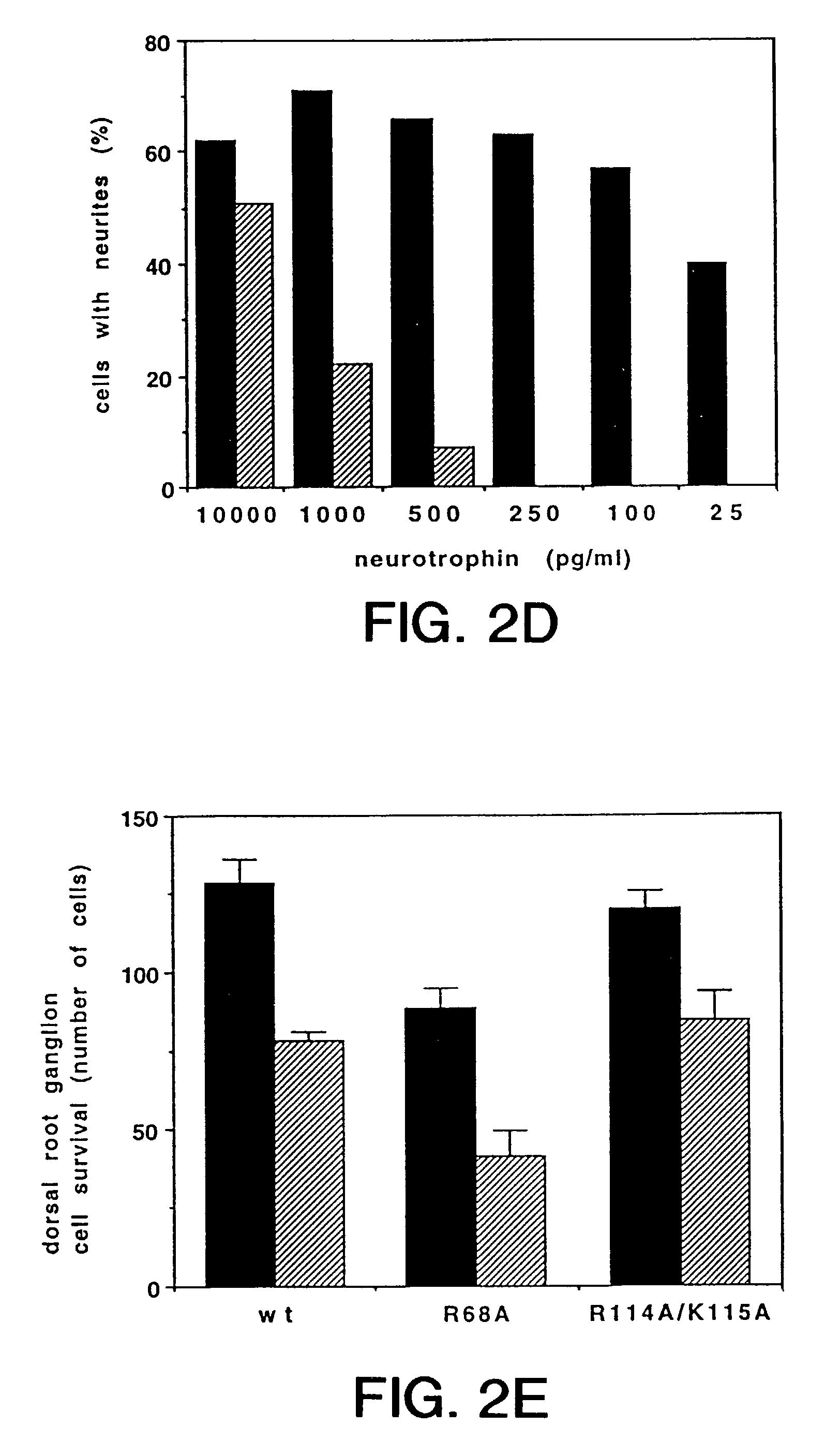
Methods of treating peripheral neuropathies using neurotrophin-3
InactiveUS6933276B1Supports survivalNervous disorderPeptide/protein ingredientsNeurotrophin-3Neurotrophic factors
The present invention relates to neurotrophin-3 (NT-3), a newly discovered member of the BDNF gene family. It is based, in part, on the identification of regions of nucleic acid sequence homology shared by BDNF and NGF (U.S. patent application Ser. No. 07 / 400,591, filed Aug. 30, 1989, incorporated by reference herein). According to the present invention, these regions of homology may be used to identify new members of the BDNF / NGF gene family; such methodology was used to identify NT-3. The present invention provides for the genes and gene products of new BDNF / NGF related neurotrophic factors identified by these methods. According to the invention, NT-3 may be used in the diagnosis and / or treatment of neurologic disorders, including, but not limited to, Alzheimer's disease and Parkinson's disease. Because NT-3 has been observed to exhibit a spectrum of activity different from the spcificities of BDNF or NGF, NT-3 provides new and valuable options for enducing regrowth and repair in the central nervous system.
Owner:REGENERON PHARM INC
Pharmaceutical formulations comprising neurotrophin mimetics
Methods and compounds for treating neurodegenerative and other disorders. Included is the administering to a subject in need thereof an effective amount of a compound having binding specificity for a p75NTR receptor molecule. Enhanced survival of neural and other cells has been observed.
Owner:U S GOVERNMENT REPRESENTED BY THE DEPT OF VETERANS AFFAIRS +2
Rescue of photoreceptors by intravitreal administration of an expression vector encoding a therapeutic protein
The invention provides methods for treating ocular diseases using a recombinant vehicle to express a protein useful in the treatment of ocular disease, with particular preference for use of neurotrophin-4 (NT4) for targeting subpopulations of cells in the retina. A genetically engineered gene transfer vector containing sequences encoding a growth factor such as neurotrophin-4 (NT4) is used to transduce cells of the retinal ganglion cell (RGC) layer, in situ, via administration of the vector intravitreally. Accordingly, methods are disclosed for treating subjects in need thereof by therapeutic protein delivery via a recombinant expression vector, including rescue of photoreceptors by targeting the RGC layer subpopulation of retinal cells.
Owner:CEREGENE
Method of treating cancer with anti-neurotrophin agents
A method of treating or preventing cancer by administering to a mammal a therapeutically effective amount of at least one anti-neurotrophin agent is described. The anti-neurotrophin agent is preferably an anti-neurotrophin antibody, an antisense molecule directed to a neurotrophin, a small organic molecule which binds a neurotrophin, or a dominant-negative mutation of a trk receptor that binds a neurotrophin. This method is particularly preferred for the treatment of prostate or pancreatic cancer. The anti-neurotrophic agents neutralize NGF, BDNF, NT-3, NT-4 / 5, NT-6 or NT-7 and include humanized antibodies as well as fragments thereof.
Owner:CEPHALON INC
Methods for therapy of neurodegenerative disease of the brain
InactiveUS20040141953A1Reduce adverse effectsAvoid restrictionsBiocideVirusesCholinergic cellsMammal
A specific clinical protocol for use toward therapy of defective, diseased and damaged cholinergic neurons in the mammalian brain, of particular usefulness for treatment of neurodegenerative conditions such as Alzheimer's disease. The protocol is practiced by delivering a definite concentration of recombinant neurotrophin into, or within close proximity of, identified defective, diseased or damaged brain cells. Using a viral vector, the concentration of neurotrophin delivered as part of a neurotrophic composition varies from 10<10 >to 10<15 >neurotrophin encoding viral particles / ml of composition fluid. Each delivery site receives from 2.5 mul to 25 mul of neurotrophic composition, delivered slowly, as in over a period of time ranging upwards of 10 minutes / delivery site. Each delivery site is at, or within 500 mum of, a targeted cell, and no more than about 10 mm from another delivery site. Stable in situ neurotrophin expression can be achieved for 12 months, or longer.
Owner:RGT UNIV OF CALIFORNIA
Agonists of Neurotrophin Receptors and Their Use as Medicaments
ActiveUS20120052094A1Preventing and treating nerve cell deathPreventing and treating and damageOrganic active ingredientsSenses disorderNeurotrophin Receptor p75Medicine
The invention relates to compounds of Formula I:and pharmaceutically acceptable salts and prodrugs thereof, wherein R1, R2, and R3 are defined as set forth in the specification. The compounds are agonists of neurotrophin (such as nerve growth factor) receptors.
Owner:CONSEJO SUPERIOR DE INVESTIGACIONES CIENTIFICAS CSIC UNDIVIDED 20 INTEREST +1
Pharmacological modulation of positive ampa receptor modulator effects on neurotrophin expression
InactiveUS20090192199A1Good effectImprove survivalBiocideNervous disorderBrain-derived neurotrophic factorFactor ii
Antagonists of group 1 metabotropic glutamate receptors (mGluR) potentiate the effect of positive AMPA receptor modulators on neurotrophin expression, such as brain-derived neurotrophic factor (BDNF). The findings described herein suggest a combinatorial approach for drug therapies, using both positive AMPA receptor modulators and mGluR antagonists. to enhance brain neurotrophism.
Owner:RGT UNIV OF CALIFORNIA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com