Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
31 results about "Cycloplegic agent" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
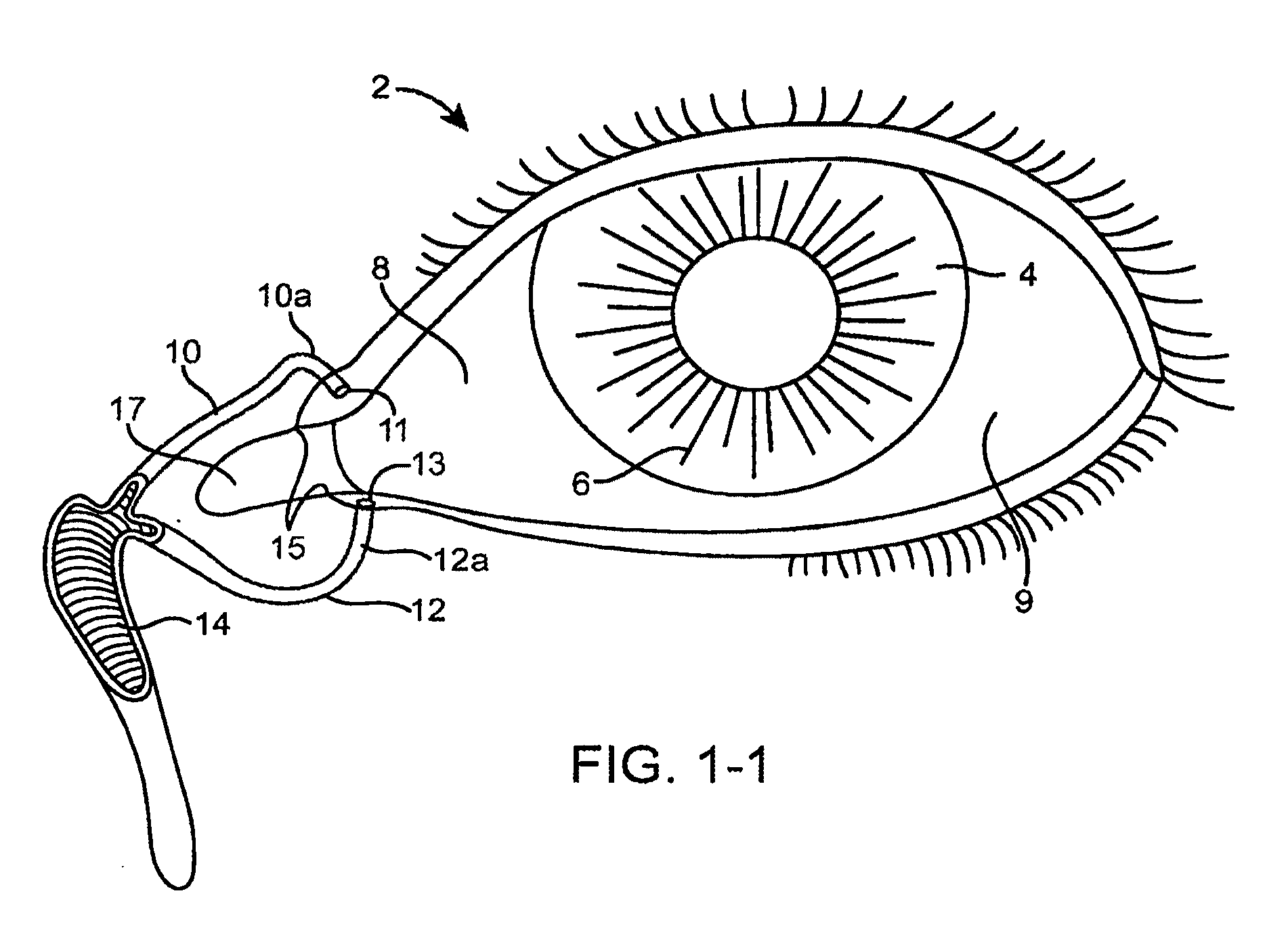
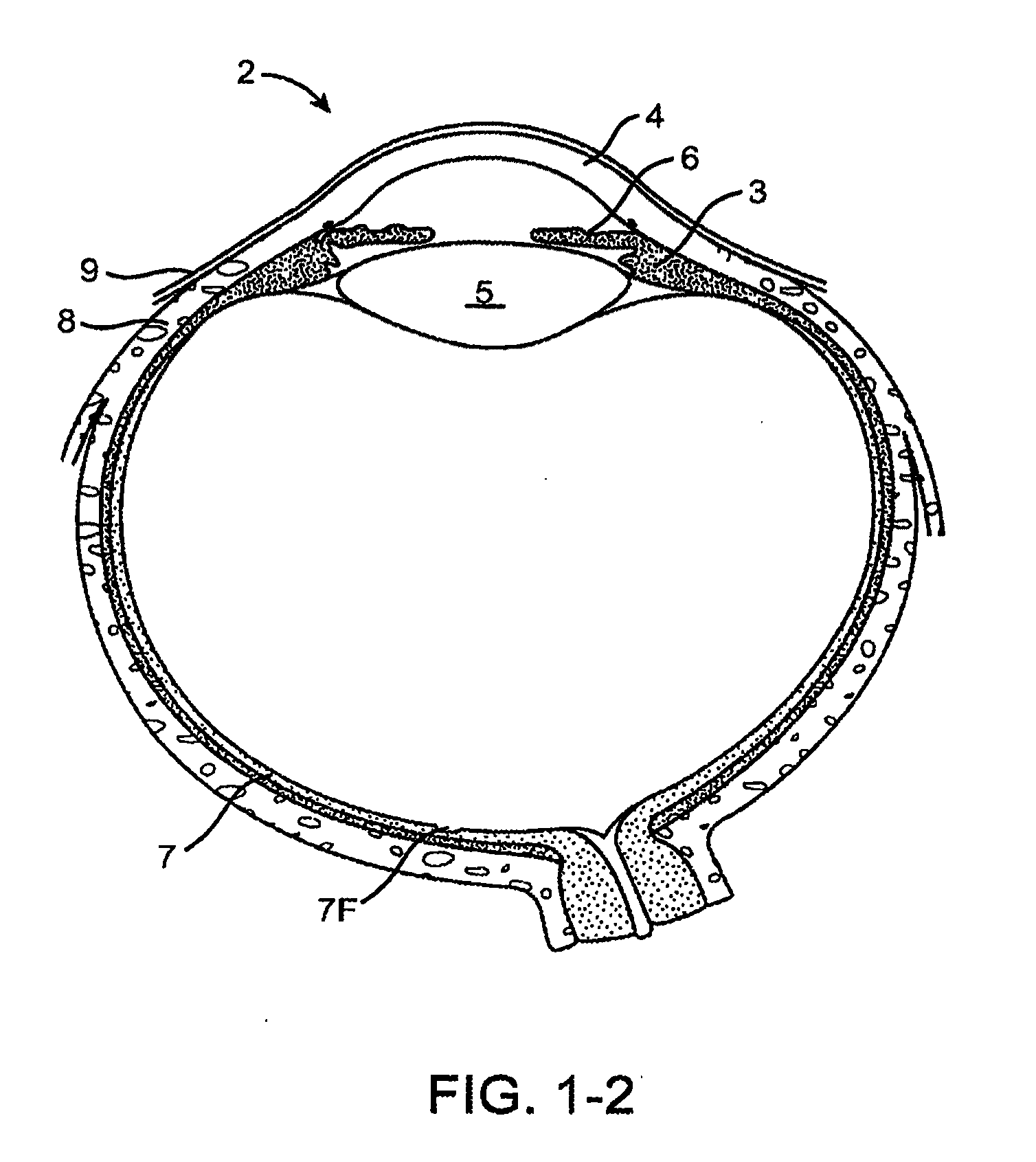
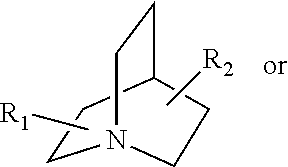
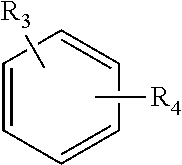
Mydriatic and cycloplegic agent. any one of several ophthalmic pharmaceutic preparations that dilate the pupil and paralyze the ocular muscles of accommodation. Mydriatics stimulate alpha adrenergic receptors or block cholinergic muscarinic receptors in the eye, temporarily paralyzing the iris sphincter muscle so that the pupil is maximally dilated.
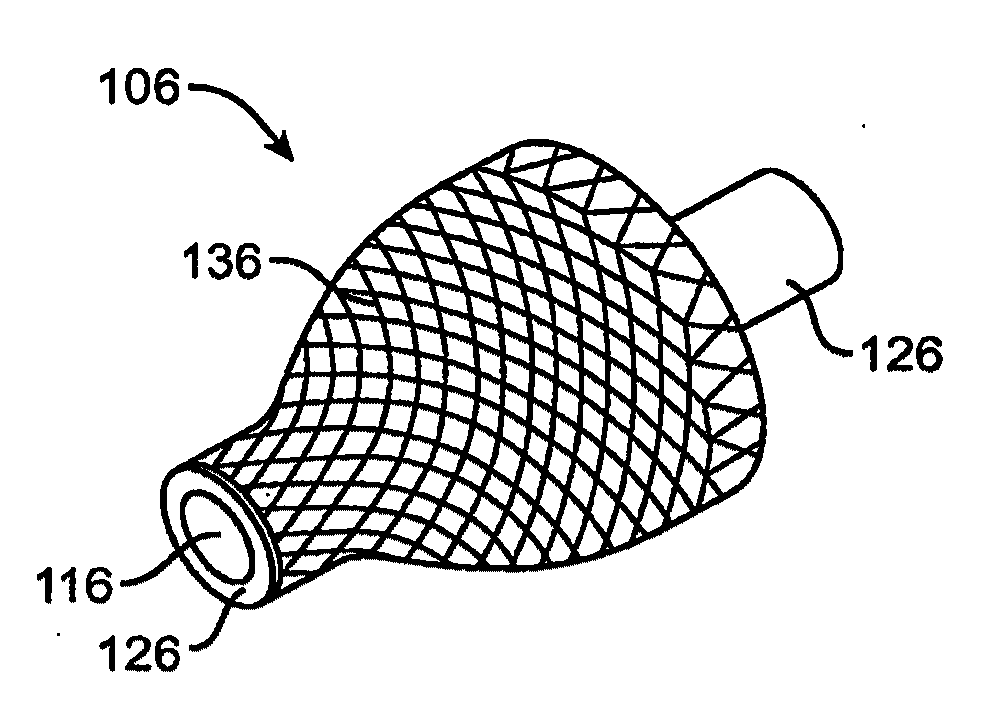
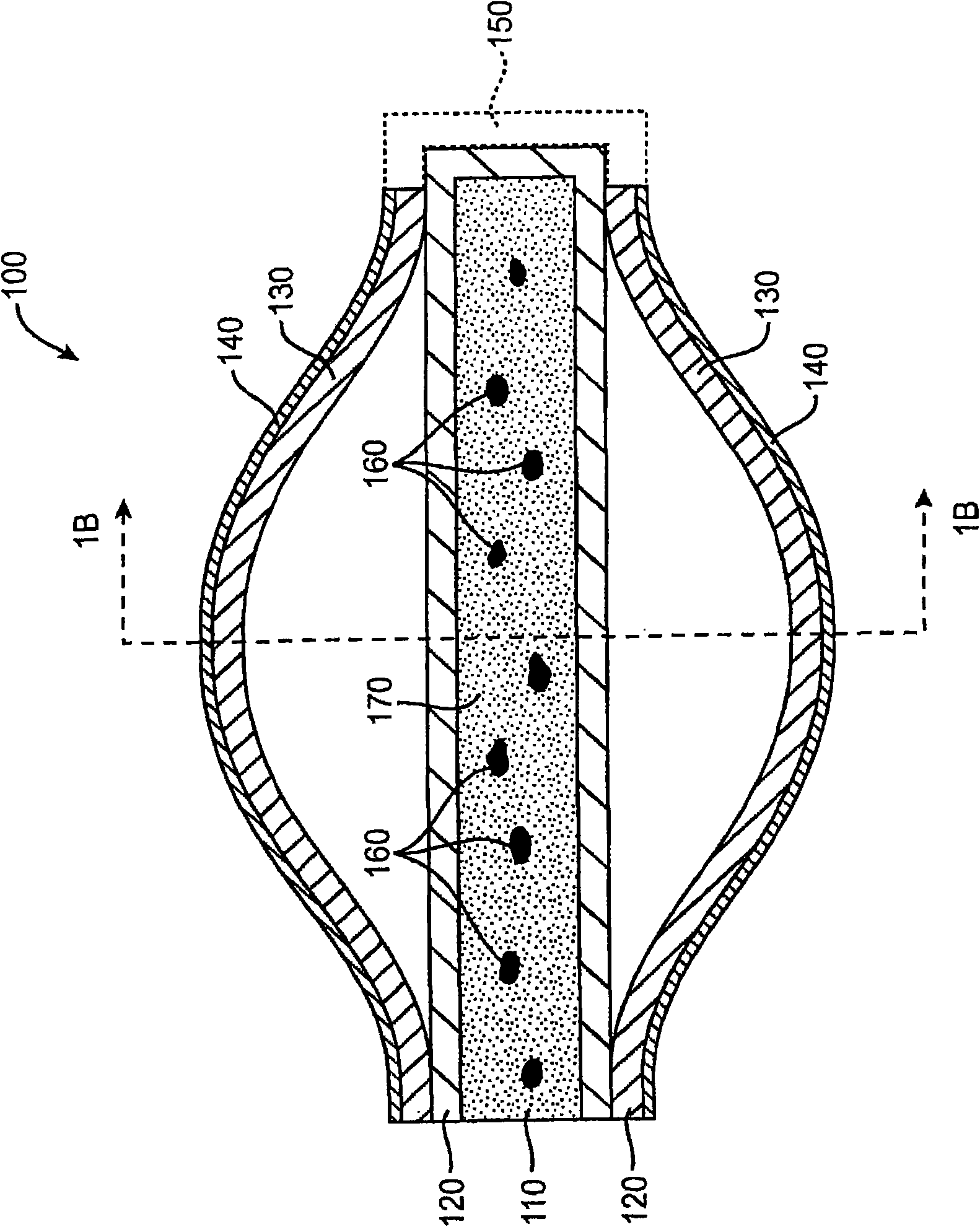
Drug delivery implants for inhibition of optical defects
InactiveUS20100114309A1Avoid side effectsInhibition releaseSenses disorderEye surgeryFar-sightednessHomatropine
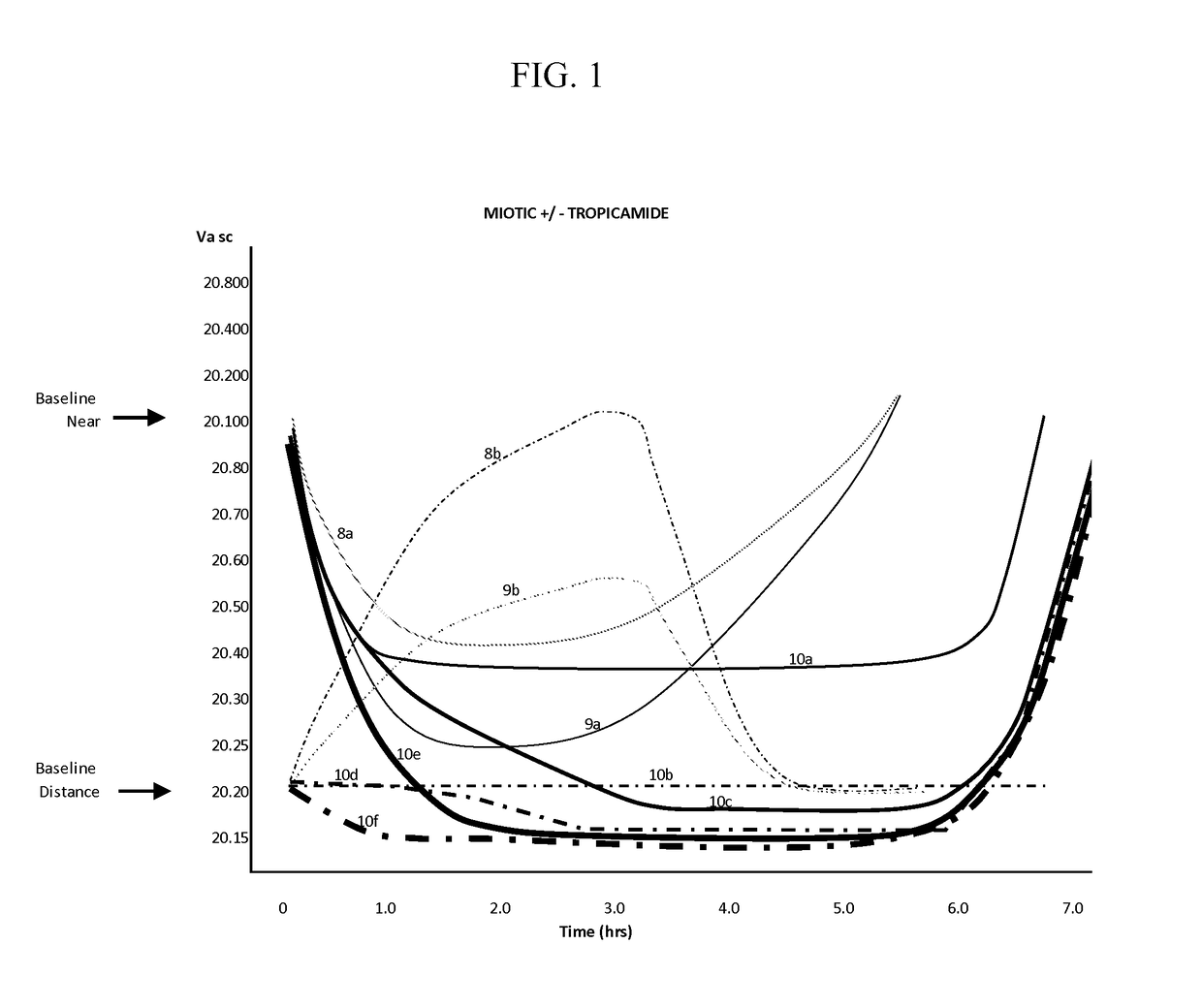
An implant for use with an eye comprises an implantable structure and a therapeutic agent. The therapeutic agent is deliverable from the structure into the eye so as to therapeutically effect and / or stabilize a refractive property of the eye. In many embodiments, the refractive property of the eye may comprise at least one of myopia, hyperopia or astigmatism. The therapeutic agent can comprise a composition that therapeutically effects or stabilizes the refractive property of the eye. The therapeutic agent may comprise at least one of a mydriatic or a cycloplegic drug. For example, the therapeutic agent may include a cycloplegic that comprises at least one of atropine, cyclopentolate, succinylcholine, homatropine, scopolamine, or tropicamide. In many embodiments, a retention element can be attached to the structure to retain the structure along a natural tissue surface.
Owner:MATI THERAPEUTICS
Compositions and Methods for the Treatment of Presbyopia
ActiveUS20150065511A1Eliminating optical aberrationImprove visual acuityBiocideSenses disorderAdrenergicMedicine
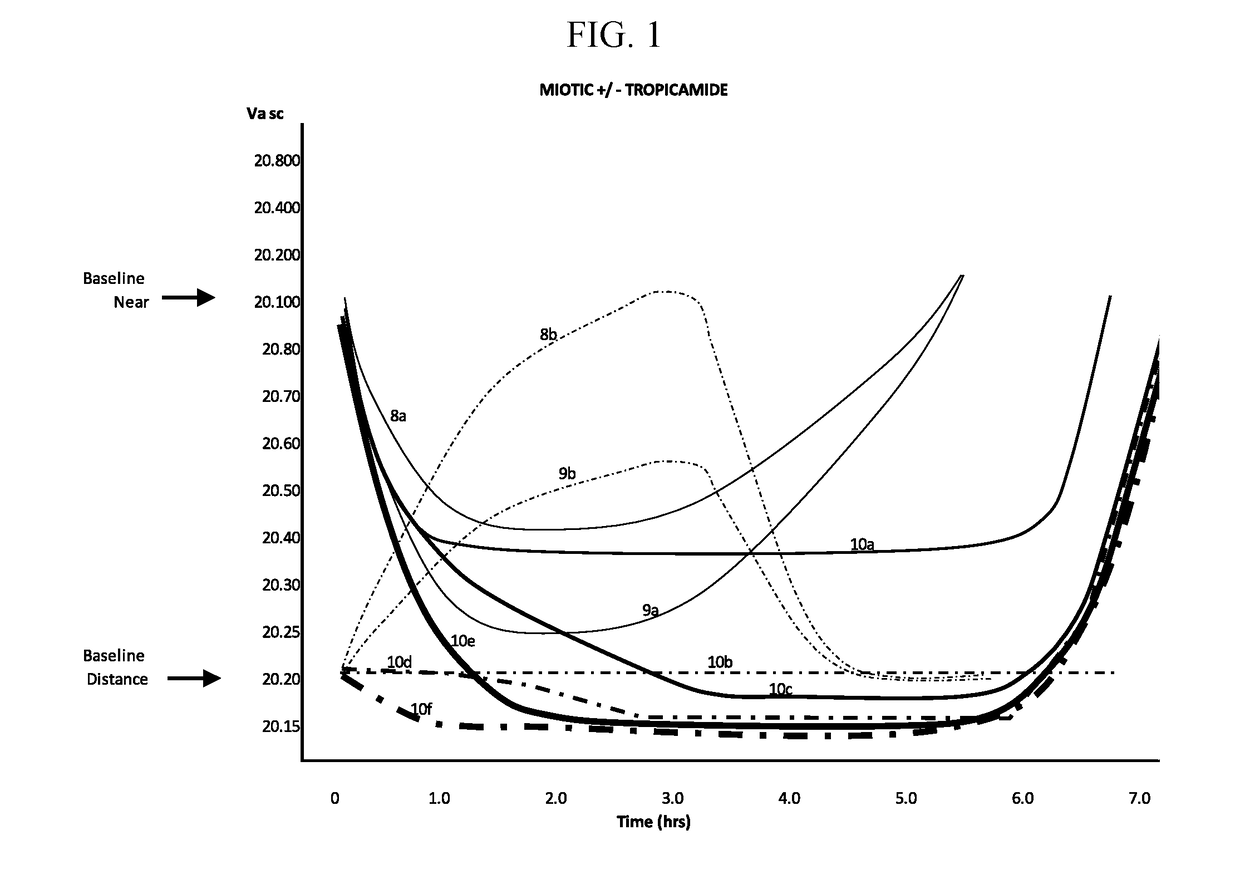
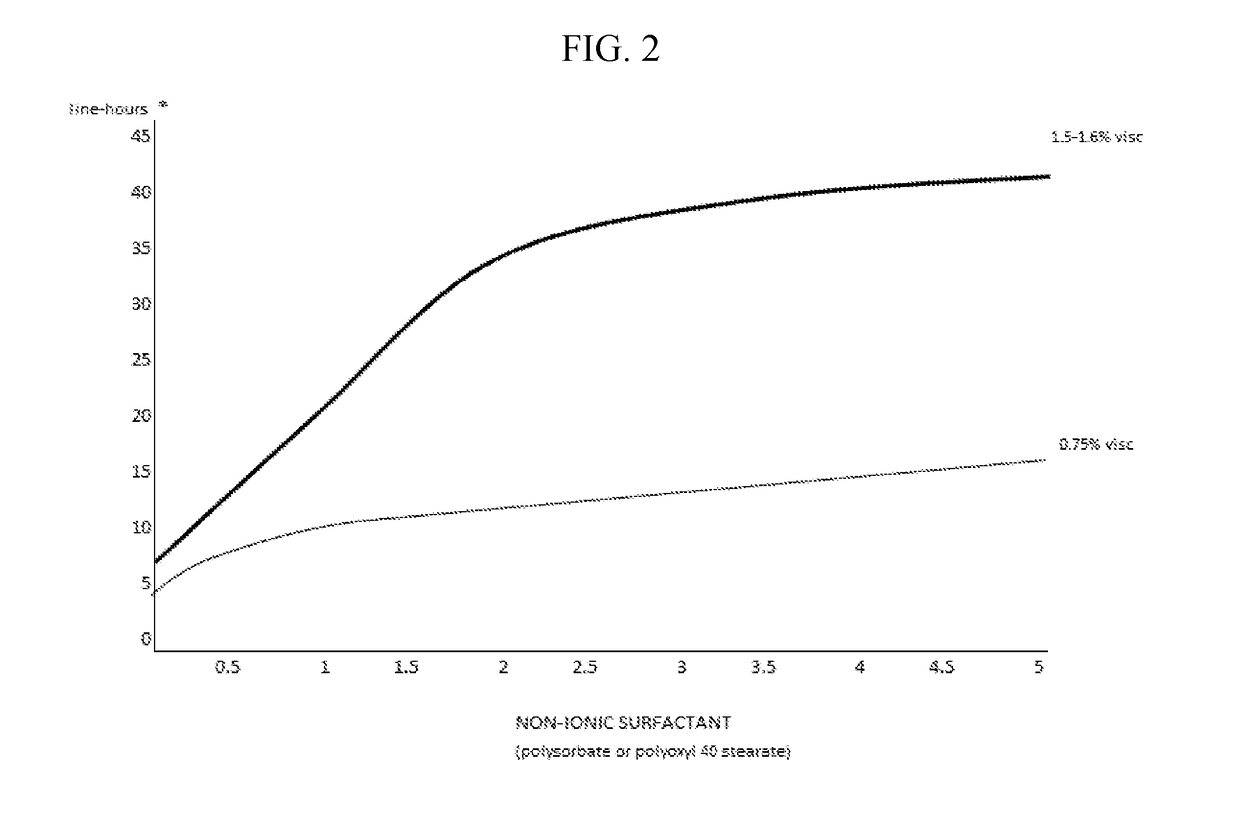
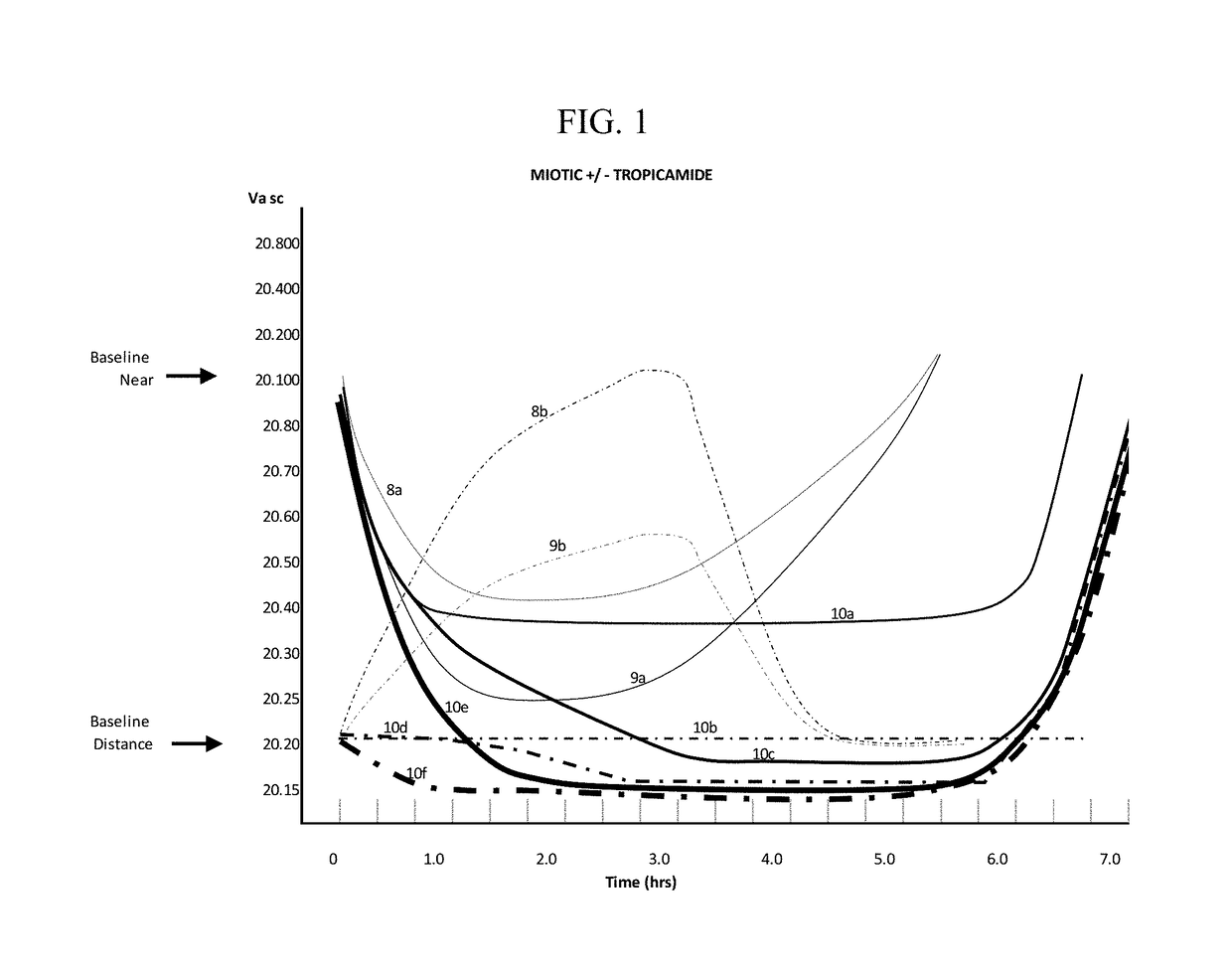
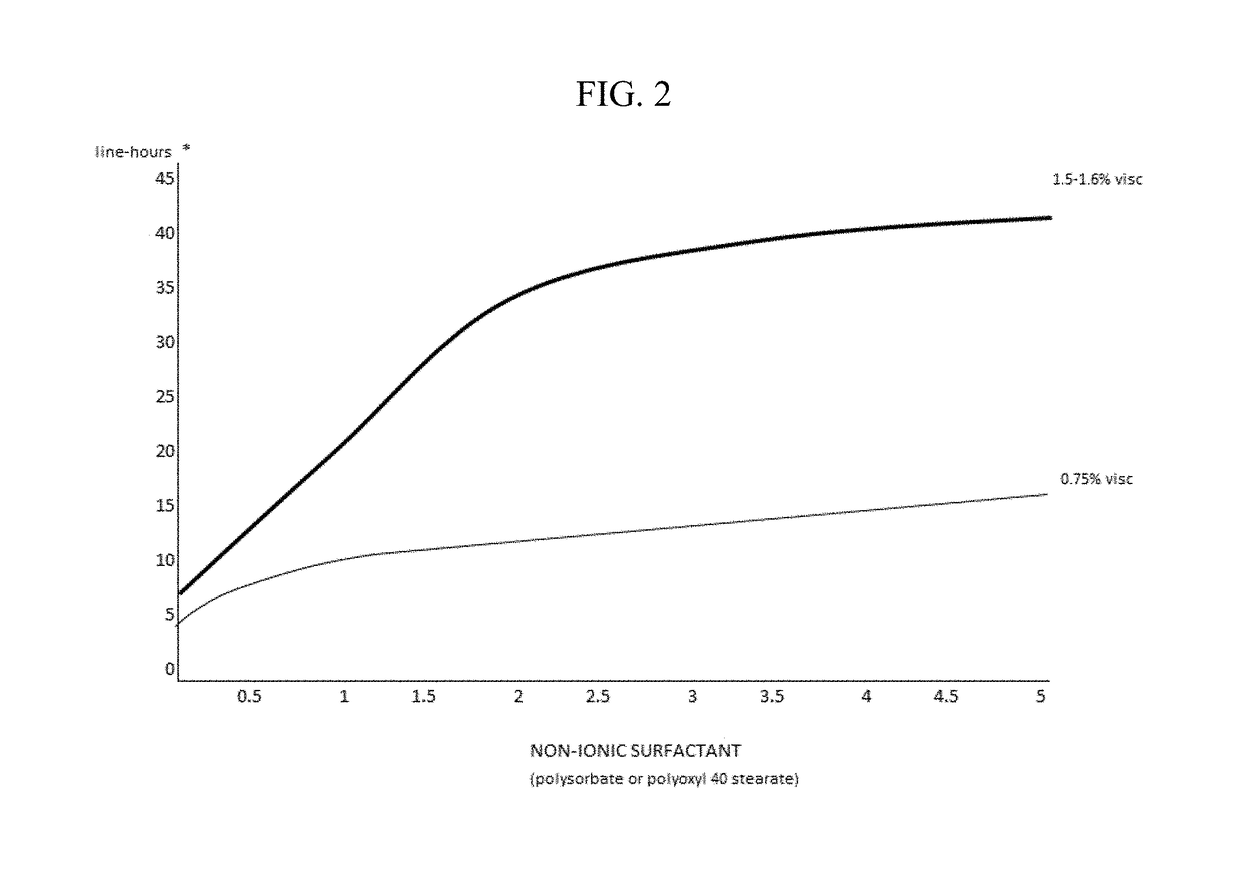
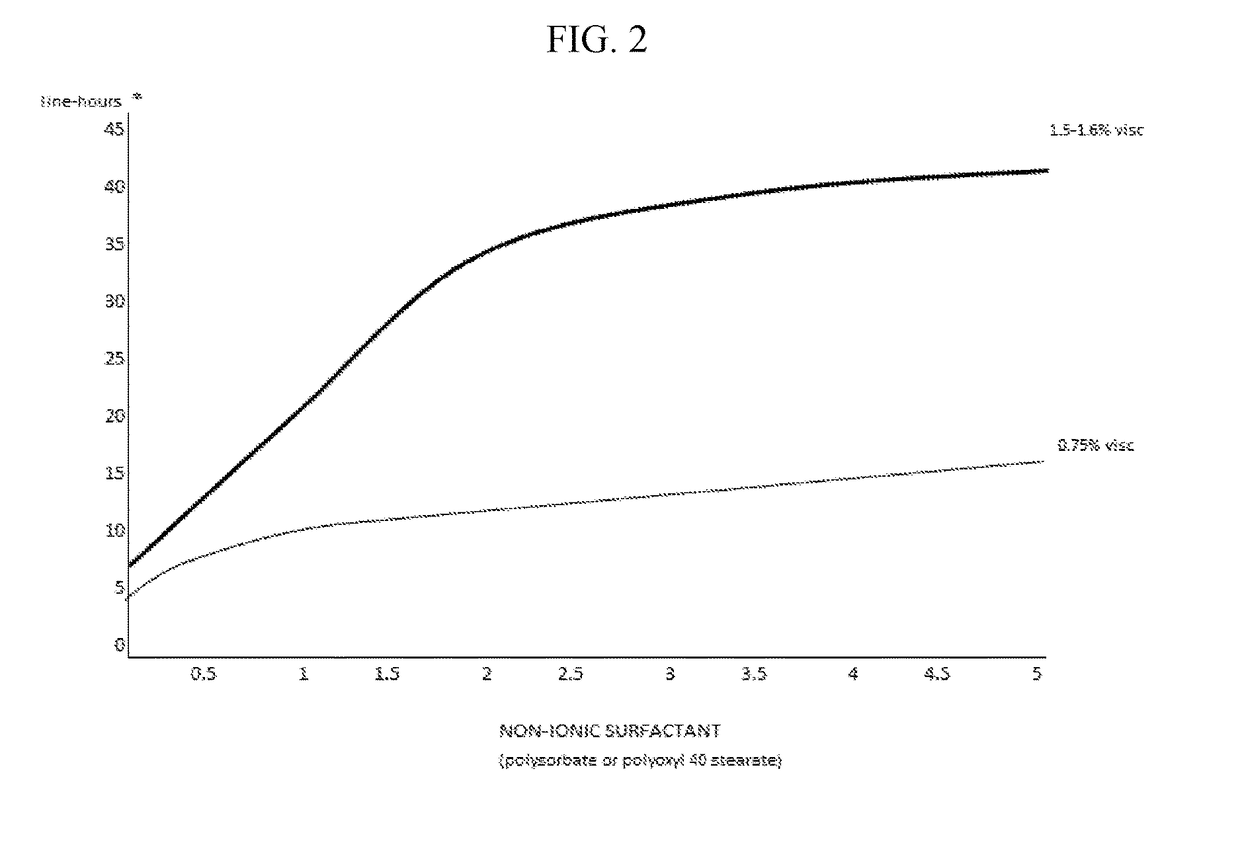
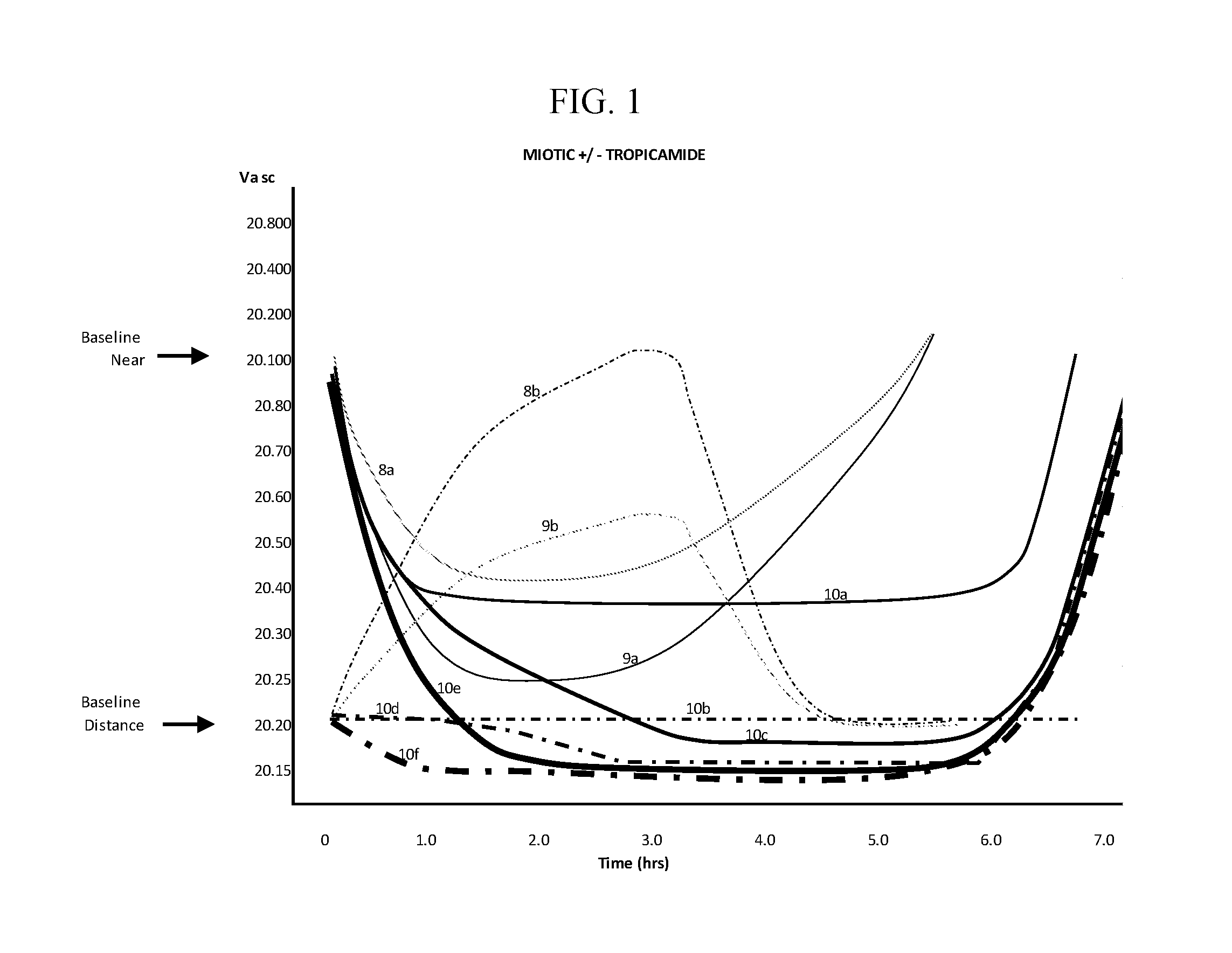
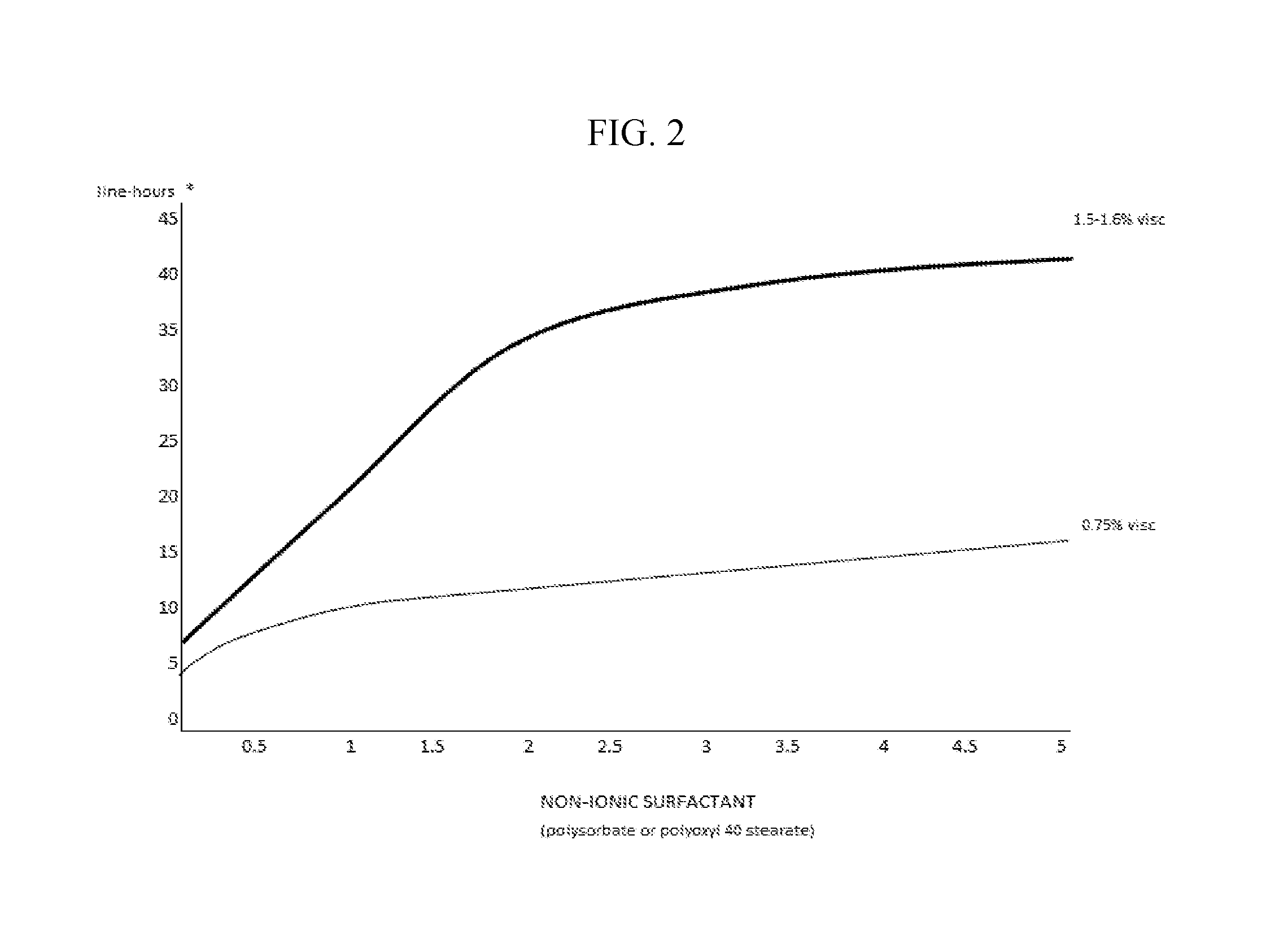
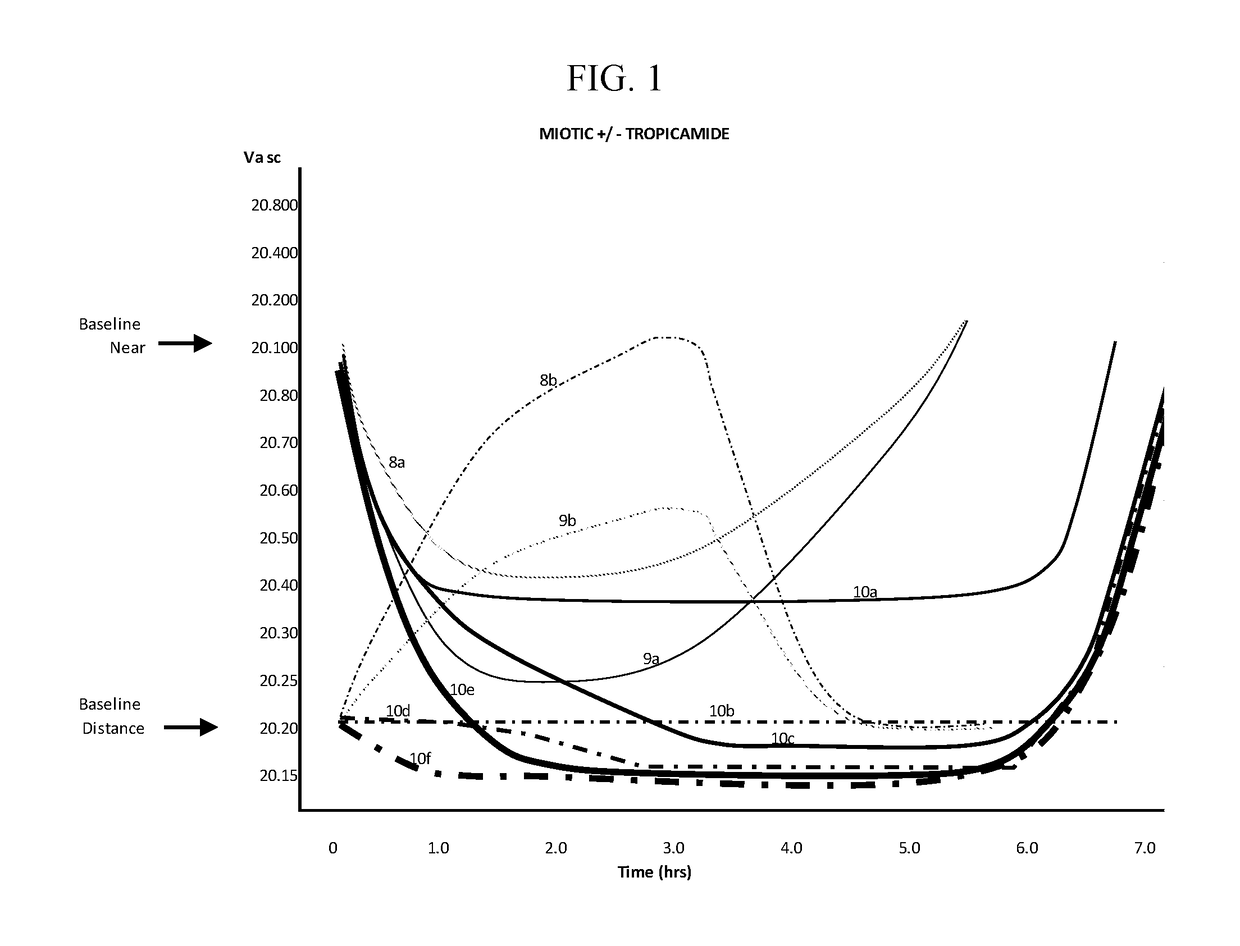
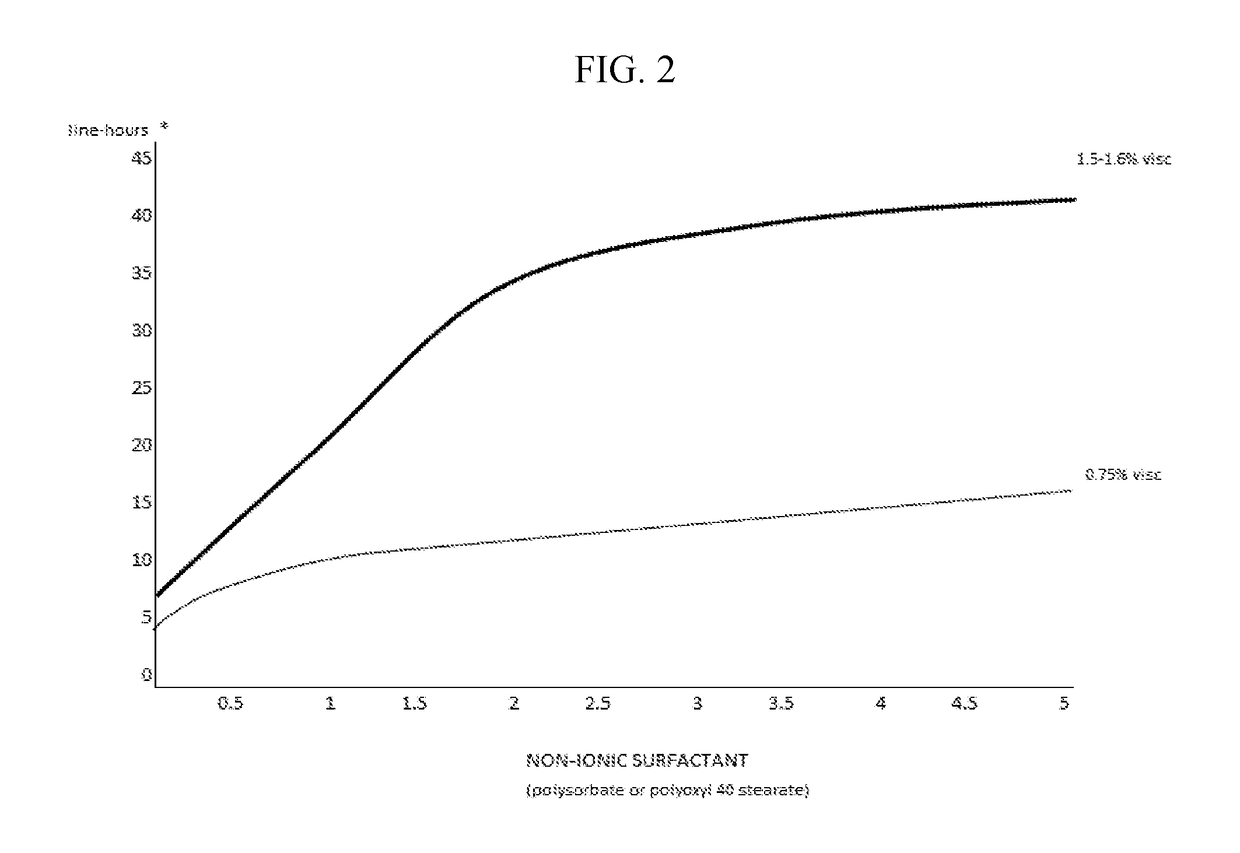
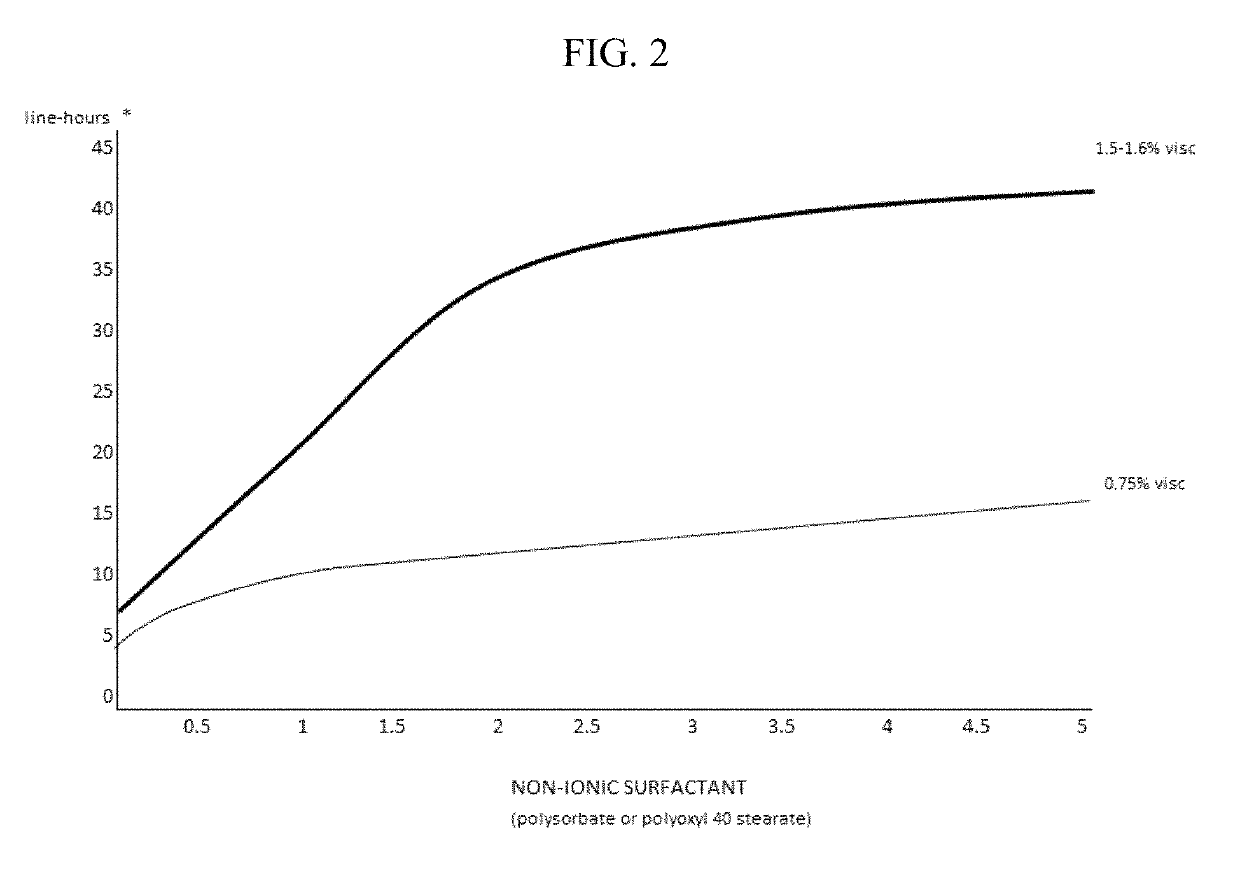
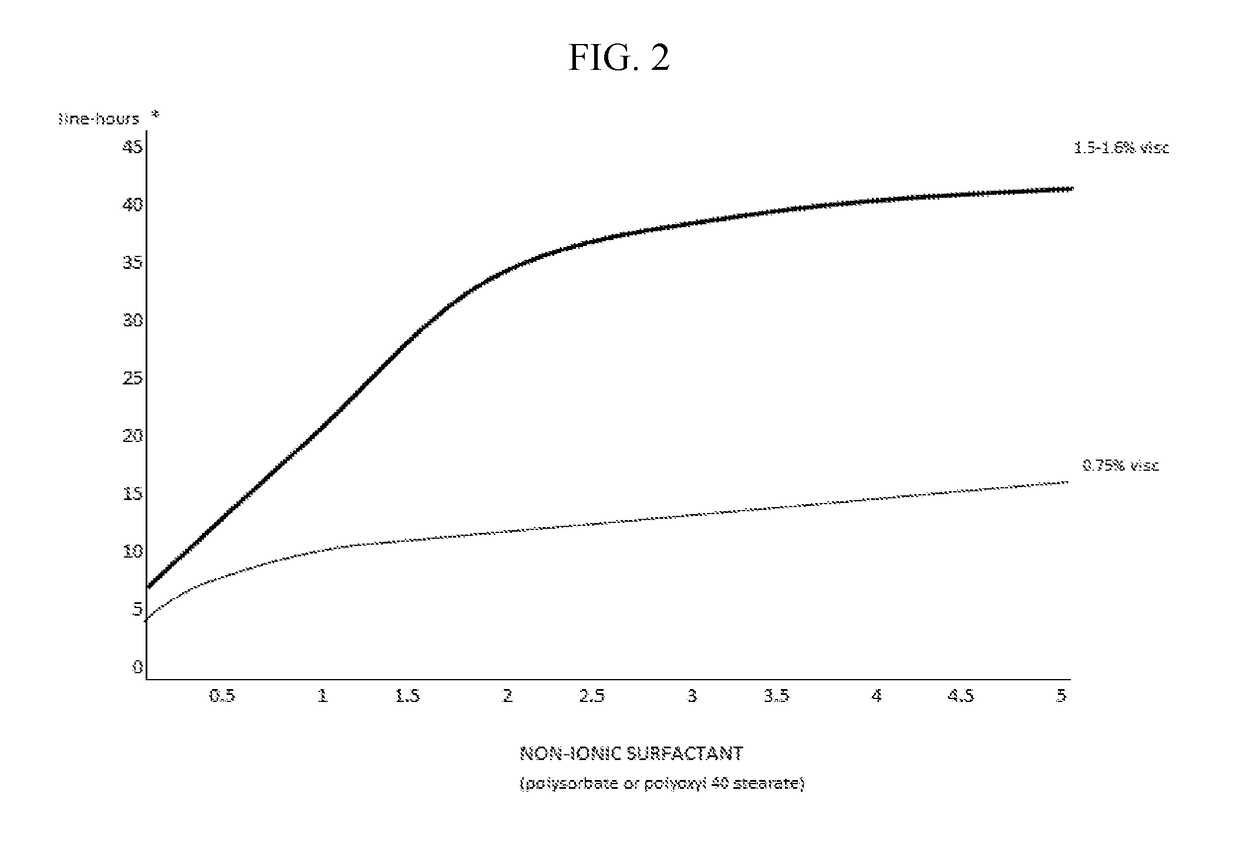
The invention provides compositions and methods for the treatment of presbyopia. The compositions preferably use aceclidine separate or together with a cycloplegic agent and / or with a nonionic surfactant and viscosity enhancer, and or with low concentrations of a selective α-2 adrenergic receptor agonist.
Owner:LENZ THERAPEUTICS INC
Storage Stable Compositions and Methods for the Treatment of Refractive Errors of the Eye
ActiveUS20150290125A1Eliminating optical aberrationImprove visual acuityBiocideInorganic non-active ingredientsRefractive errorOphthalmology
The invention provides compositions and methods for achieving storage stable aceclidine. The compositions preferably comprise aceclidine, a cycloplegic agent, a surfactant, a tonicity adjustor and optionally a viscosity enhancer and an antioxidant. The invention further provides methods for treating refractive errors of the eye with a storage stable aceclidine composition.
Owner:LENZ THERAPEUTICS INC
Drug delivery implants for inhibition of optical defects
An implant for use with an eye comprises an implantable structure and a therapeutic agent. The therapeutic agent is deliverable from the structure into the eye so as to therapeutically effect and / or stabilize a refractive property of the eye. In many embodiments, the refractive property of the eye may comprise at least one of myopia, hyperopia or astigmatism. The therapeutic agent can comprise a composition that therapeutically effects or stabilizes the refractive property of the eye. The therapeutic agent may comprise at least one of a mydriatic or a cycloplegic drug. For example, the therapeutic agent may include a cycloplegic that comprises at least one of atropine, cyclopentolate, succinylcholine, homatropine, scopolamine, or tropicamide. In many embodiments, a retention element can be attached to the structure to retain the structure along a natural tissue surface.
Owner:QLT INC
Compositions and methods for the treatment of presbyopia
ActiveUS9089562B2Increasing visual depth of fieldLong durationOrganic active ingredientsSenses disorderAdrenergicMedicine
The invention provides compositions and methods for the treatment of presbyopia. The compositions preferably use aceclidine separate or together with a cycloplegic agent and / or with a nonionic surfactant and viscosity enhancer, and or with low concentrations of a selective α-2 adrenergic receptor agonist.
Owner:LENZ THERAPEUTICS INC
Compositions and methods for the treatment of presbyopia
ActiveUS9968594B2Organic active ingredientsPharmaceutical delivery mechanismPhysiologyCycloplegic agent
The invention provides compositions and methods for the treatment of presbyopia. In a preferred embodiment correction of presbyopia occurs without reduction in distance vision acuity. The compositions of the invention preferably contain a muscarinic agonist and a cycloplegic agent.
Owner:LENZ THERAPEUTICS INC
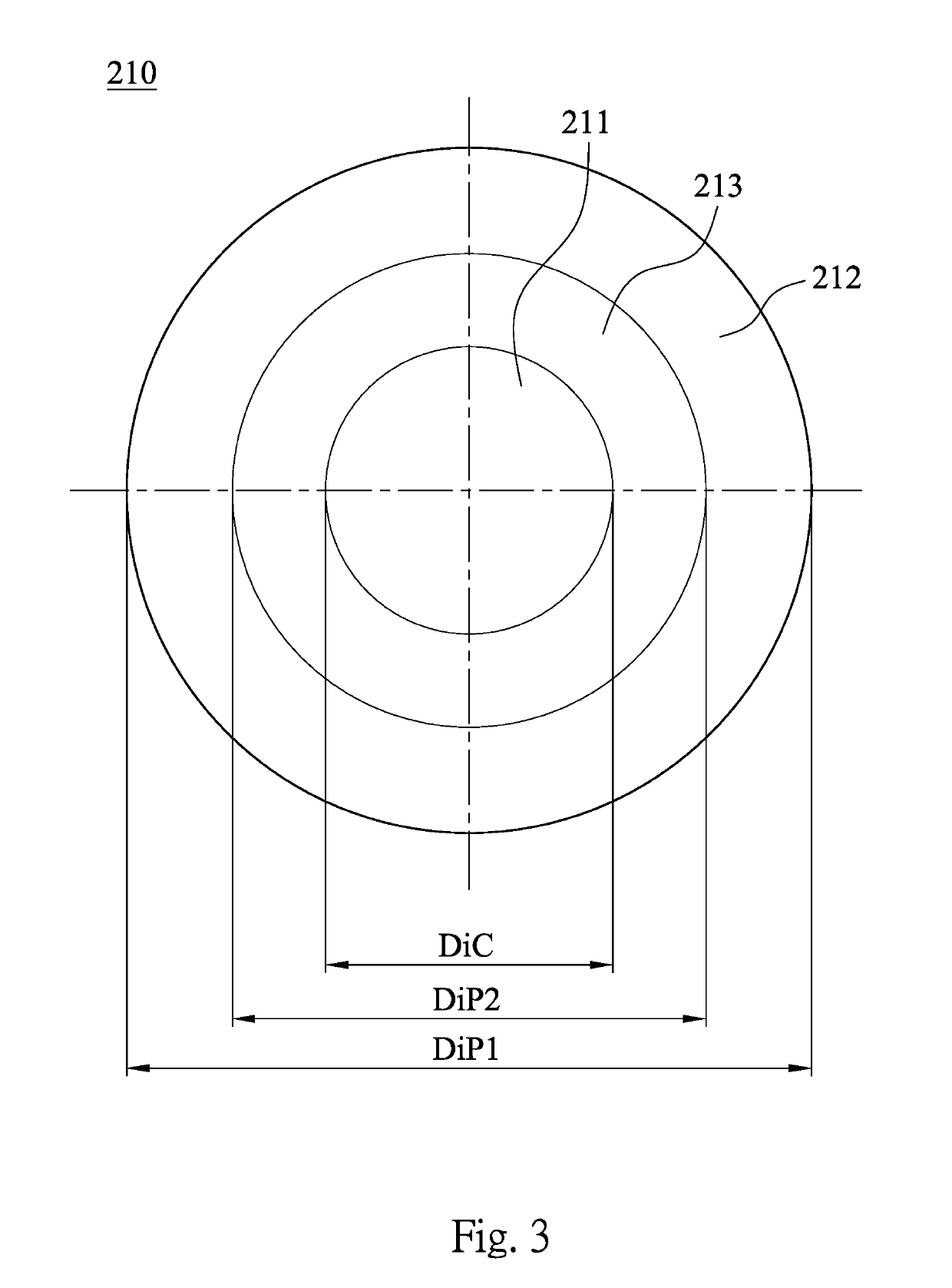
Contact lens product
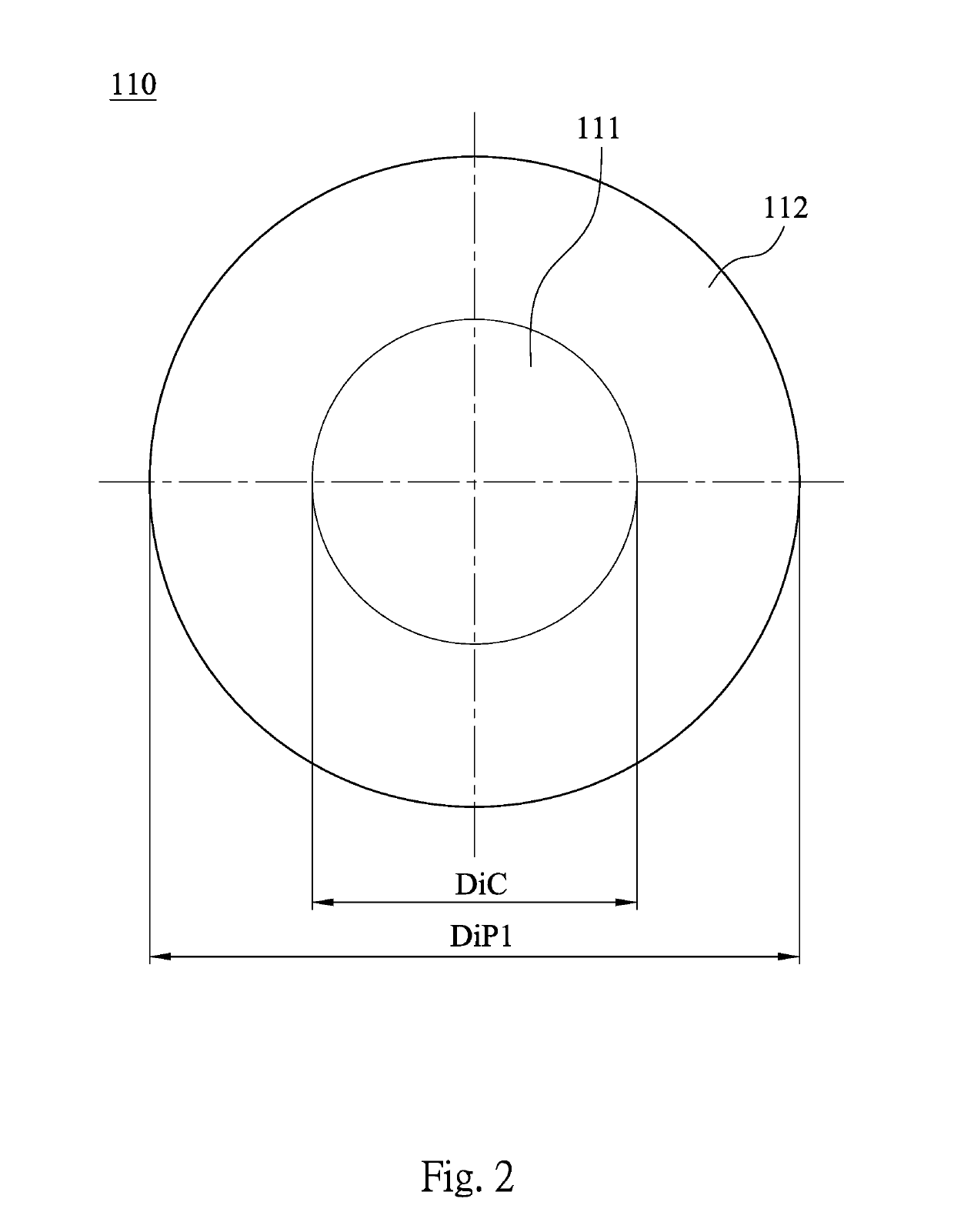
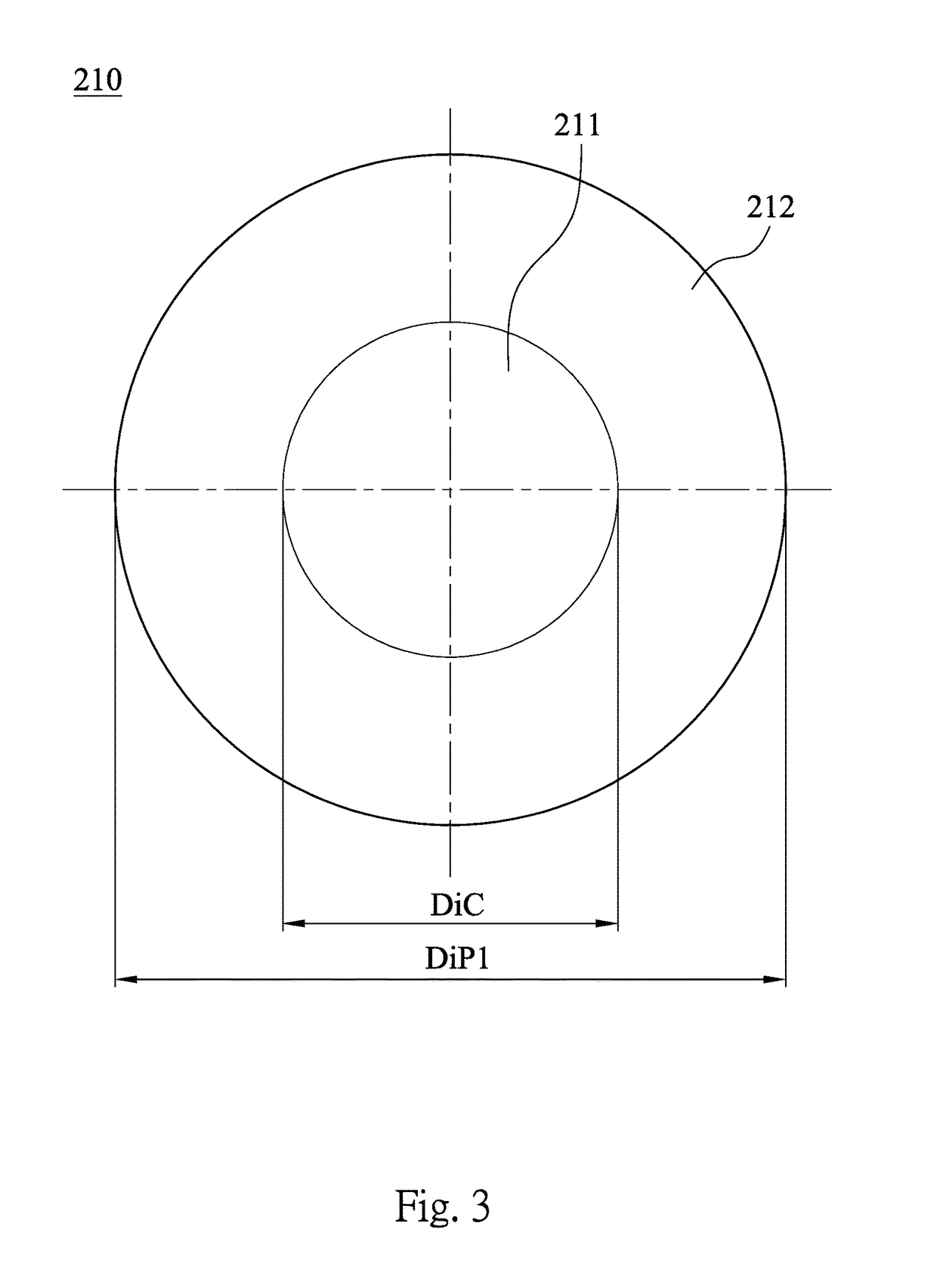
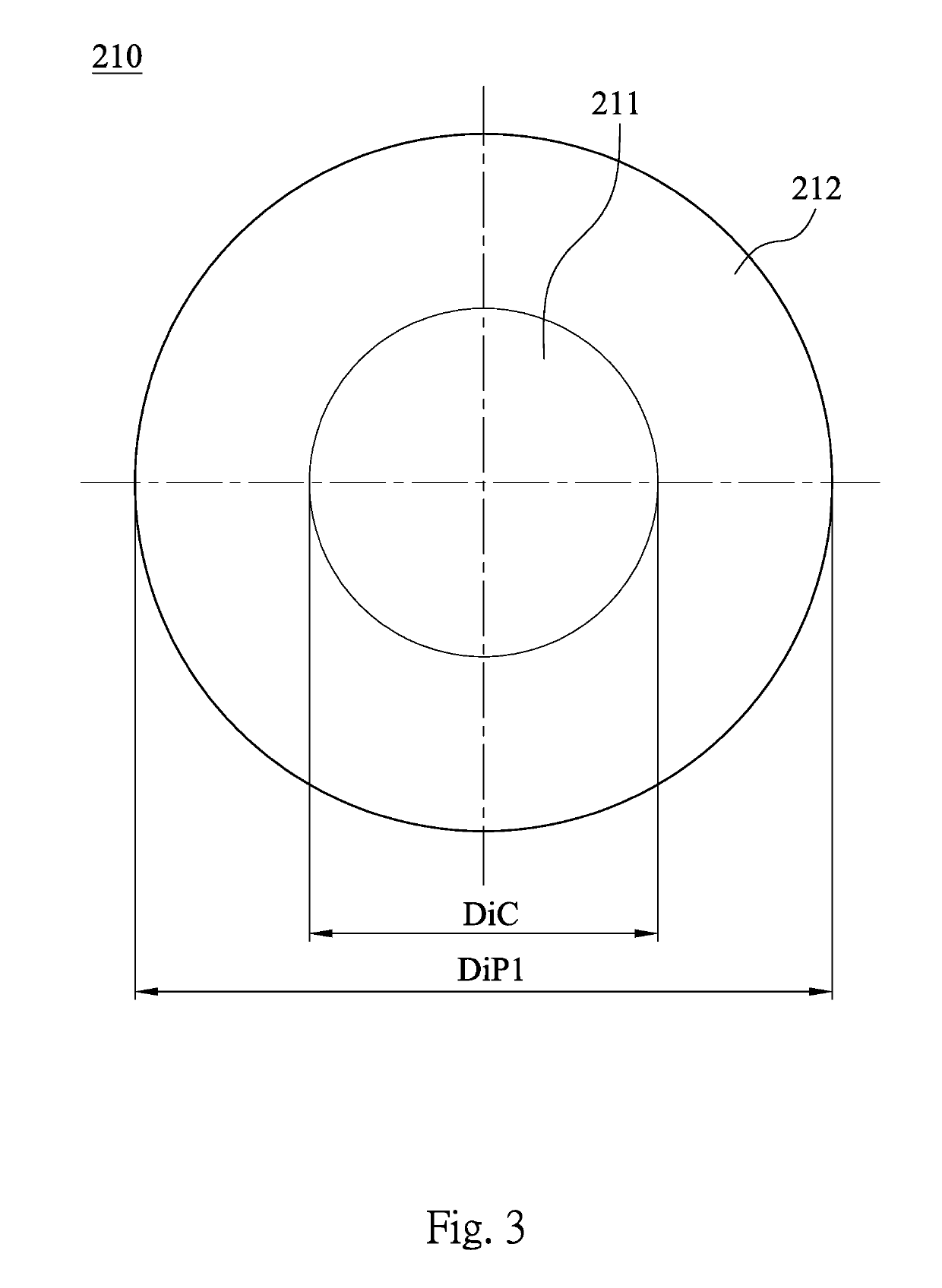
A contact lens product includes a multifocal contact lens and a buffer solution. The multifocal contact lens includes a central region and at least one annular region. The annular region concentrically surrounds the central region. A diopter of the annular region is different from a diopter of the central region. The multifocal contact lens is immersed in the buffer solution, and the buffer solution includes a cycloplegic agent.
Owner:LARGAN MEDICAL CO LTD
Compositions and Methods for the Improvement of Distance Vision and the Treatment of Refractive Errors of the Eye
ActiveUS20150290126A1Eliminating optical aberrationImprove visual acuityBiocideInorganic non-active ingredientsRefractive errorCryoprecipitate
The invention provides compositions and methods for the improvement of distance vision. The invention further provides compositions and methods for the treatment of refractive errors of the eye. The invention further provides compositions that preferably comprise aceclidine lyophilized with a cryoprecipitate separate or together with a diluent comprising a cycloplegic agent, a surfactant, and optionally a viscosity enhancer.
Owner:LENZ THERAPEUTICS INC
Contact lens product
A contact lens product includes a contact lens and a buffer solution. The contact lens is immersed in the buffer solution, and the buffer solution includes a cycloplegic agent.
Owner:LARGAN MEDICAL CO LTD
Compositions and methods for the improvement of distance vision and the treatment of refractive errors of the eye
ActiveUS9314427B2Organic active ingredientsPharmaceutical delivery mechanismRefractive errorCryoprecipitate
The invention provides compositions and methods for the improvement of distance vision. The invention further provides compositions and methods for the treatment of refractive errors of the eye. The invention further provides compositions that preferably comprise aceclidine lyophilized with a cryoprecipitate separate or together with a diluent comprising a cycloplegic agent, a surfactant, and optionally a viscosity enhancer.
Owner:LENZ THERAPEUTICS INC
Compositions and Methods for the Treatment of Presbyopia
ActiveUS20160008337A1Without reducing distance vision acuityBiocidePharmaceutical delivery mechanismPhysiologyCycloplegic agent
The invention provides compositions and methods for the treatment of presbyopia. In a preferred embodiment correction of presbyopia occurs without reduction in distance vision acuity. The compositions of the invention preferably contain a muscarinic agonist and a cycloplegic agent.
Owner:LENZ THERAPEUTICS INC
Contact lens product
A contact lens product includes a multifocal contact lens and a buffer solution. The multifocal contact lens includes a central region and at least one annular region. The annular region concentrically surrounds the central region. A diopter of the annular region is different from a diopter of the central region. The multifocal contact lens is immersed in the buffer solution, and the buffer solution includes a cycloplegic agent.
Owner:LARGAN MEDICAL CO LTD
Storage stable compositions and methods for the treatment of refractive errors of the eye
ActiveUS9320709B2Organic active ingredientsPharmaceutical delivery mechanismRefractive errorOphthalmology
Owner:LENZ THERAPEUTICS INC
Non-aqueous oil delivery system for ophthalmic drugs
InactiveUS20100323978A1Comfortable to useImprove effectivenessBiocideSenses disorderWhite petrolatumVegetable oil
The present invention relates to a delivery system for ophthalmic drugs, and more particularly, to a non aqueous oil delivery system. Low concentrations of ophthalmic drugs suspended in an oil vehicle delivery system are as therapeutically effective in man and animals as the corresponding higher concentrations of ophthalmic drugs that are commercially used in aqueous solutions. Eye drops that utilize this nonaqueous oil delivery system, when used in man, are comfortable to use and produce little ocular irritation, have a longer shelf-life, low systemic toxic potential, and only short term blurring of vision. Using this nonaqueous oil delivery system, a single drop of ophthalmic drug with a concentration that is 10 times less than the same drug used in commercially available aqueous eye drops is as effective as the commercially available aqueous ophthalmic eye drops that require many applications to be effective. In addition, utilizing the nonaqueous oil delivery system as eye drops produces no ocular sensation of burning, stinging or excessive tearing that is commonly associated with the commercially available aqueous eye drops. This very dilute ophthalmic drug preparation has a greatly reduced systemic toxicity potential as compared to the commercially used aqueous ophthalmic drops. The vehicle includes castor oil, corn oil, glycerol, mineral oil USP, vegetable oil, white petrolatum USP, and mixtures, there of. The ophthalmic class of drugs includes antimicrobials, miotics, mydriatics, mydriatic-cycloplegics, mydriatic-cycloplegic reversal agents and topical anesthetics.
Owner:HANNA CALVIN
Compositions and methods for the treatment of presbyopia
ActiveUS9833441B2Increasing visual depth of fieldEliminate side effectsOrganic active ingredientsPharmaceutical delivery mechanismPreservativeDermatology
The invention provides compositions and methods for the treatment of presbyopia. The compositions preferably comprise aceclidine and a cycloplegic agent. The compositions optionally contain a surfactant, a viscosity enhancer, an osmolarity modifier and a preservative.
Owner:LENZ THERAPEUTICS INC
Bendazol eye drops
InactiveCN102525906AConfidenceQuick effectOrganic active ingredientsSenses disorderAdditive ingredientGlycerol
The invention discloses bendazol eye drops, which are prepared by mixing components including bendazol, sodium chloride, glycerol and ethylparaben and then adding water for injection. The bendazol eye drops can overcome the defects that cycloplegic used for mydriatic treatment in the traditional pseudomyopia treating manner at home and abroad causes reduced ciliary muscle tension, lasting mydriasis, phengophobia and lacrimation, photophobia discomfort and the like, and be directly dropped into eyes to relax smooth muscles (including ciliary muscles) and expand anterior ciliary arteries and veins, without mydriasis, so as to achieve a treatment function.
Owner:轩红军
Contact lens compositions and methods for the treatment of presbyopia
The invention provides contact lens and contact lens storage compositions and methods for the treatment of presbyopia. The contact lens and contact lens storage compositions preferably comprise aceclidine and a cryoprotectant. The compositions optionally contain a cycloplegic agent.
Owner:LENZ THERAPEUTICS INC
Compositions and methods for the treatment of presbyopia
ActiveUS9844537B2Improve visionImprove eyesightHydroxy compound active ingredientsPharmaceutical delivery mechanismPolyolPreservative
The invention provides compositions and methods for the treatment of presbyopia. The compositions preferably comprise aceclidine and a polyol. The compositions optionally contain a cycloplegic agent, a surfactant, a viscosity enhancer, an osmolarity modifier and a preservative.
Owner:LENZ THERAPEUTICS INC
Compositions and methods for the treatment of presbyopia
ActiveUS10617763B2High viscosityReduce rednessOrganic active ingredientsSenses disorderPreservativeAceclidine
The invention provides compositions and methods for the treatment of presbyopia. The compositions preferably comprise aceclidine and a cryoprotectant. The compositions optionally contain a cycloplegic agent, a surfactant, a viscosity enhancer, an osmolarity modifier and a preservative.
Owner:LENZ THERAPEUTICS INC
Compositions and Methods for the Treatment of Presbyopia
ActiveUS20160346259A1Eliminating optical aberrationImprove visual acuityOrganic active ingredientsPharmaceutical delivery mechanismPolyolPreservative
The invention provides compositions and methods for the treatment of presbyopia. The compositions preferably comprise aceclidine and a polyol. The compositions optionally contain a cycloplegic agent, a surfactant, a viscosity enhancer, an osmolarity modifier and a preservative.
Owner:LENZ THERAPEUTICS INC
Compositions and methods for the treatment of presbyopia
ActiveUS10052313B2High viscosityReduce rednessOrganic active ingredientsPharmaceutical delivery mechanismPolyolPreservative
The invention provides compositions and methods for the treatment of presbyopia. The compositions preferably comprise aceclidine and a polyol. The compositions optionally contain a cycloplegic agent, a surfactant, a viscosity enhancer, an osmolarity modifier and a preservative.
Owner:LENZ THERAPEUTICS INC
Contact lens compositions and methods for the treatment of presbyopia
The invention provides contact lens and contact lens storage compositions and methods for the treatment of presbyopia. The contact lens and contact lens storage compositions preferably comprise aceclidine and a cryoprotectant. The compositions optionally contain a cycloplegic agent.
Owner:LENZ THERAPEUTICS INC
Contact lens compositions and methods for the treatment of presbyopia
InactiveUS20190240152A1Organic active ingredientsPharmaceutical delivery mechanismAceclidineCycloplegic agent
The invention provides contact lens and contact lens storage compositions and methods for the treatment of presbyopia. The contact lens and contact lens storage compositions preferably comprise aceclidine and a cryoprotectant. The compositions optionally contain a cycloplegic agent.
Owner:LENZ THERAPEUTICS INC
Storage stable compositions and methods for the treatment of refractive errors of the eye
InactiveCN107920984AImprove acuityNo need for correctionOrganic active ingredientsSenses disorderRefractive errorOphthalmology
The invention provides compositions and methods for achieving storage stable aceclidine. The compositions preferably comprise aceclidine, a cycloplegic agent, a surfactant, a tonicity adjuster and optionally a viscosity enhancer and an antioxidant. The invention further provides methods for treating refractive errors of the eye with a storage stable aceclidine composition.
Owner:伦茨治疗股份有限公司
Compositions and Methods for the Treatment of Presbyopia
ActiveUS20180140708A1Eliminating optical aberrationImprove visual acuityOrganic active ingredientsSenses disorderPreservativeViscosity
The invention provides compositions and methods for the treatment of presbyopia. The compositions preferably comprise aceclidine and a cryoprotectant. The compositions optionally contain a cycloplegic agent, a surfactant, a viscosity enhancer, an osmolarity modifier and a preservative.
Owner:LENZ THERAPEUTICS INC
Eye Medication Formulation with Antibacterial Agent
InactiveUS20090156587A1Reduce or eliminate patient discomfortOrganic active ingredientsOphthalmologyAnti bacterial
A formulation for administration to the eye has at least one pharmaceutical agent such as a mydriatic agent, a cycloplegic agent, an anesthetic or a non-steroidal anti-inflammation agent combined with an anti-bacterial agent and a suitable carrier. The formulation can be made for topical or intracameral administration to the eye.
Owner:NALLAKRISHNAN RAVI
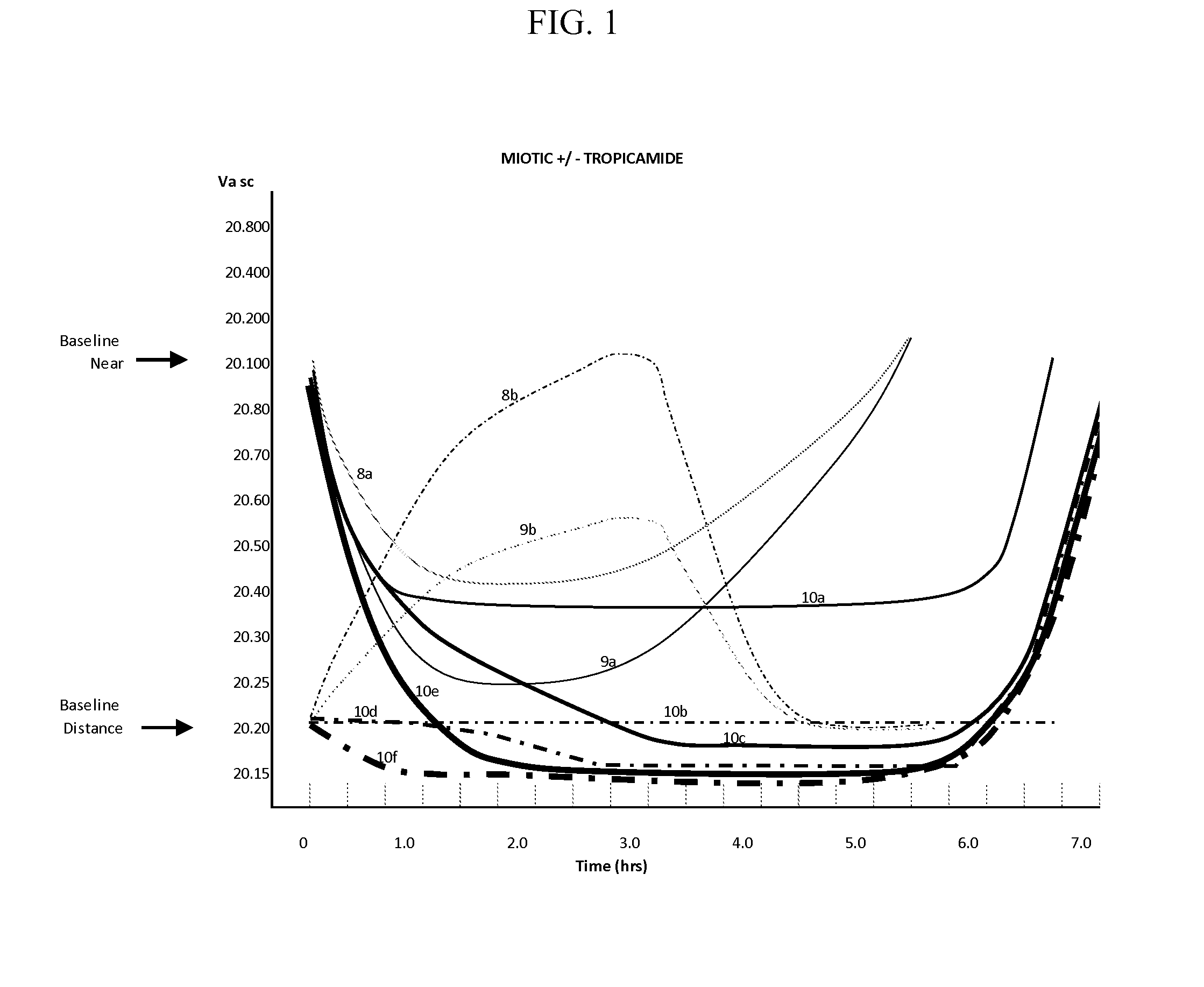
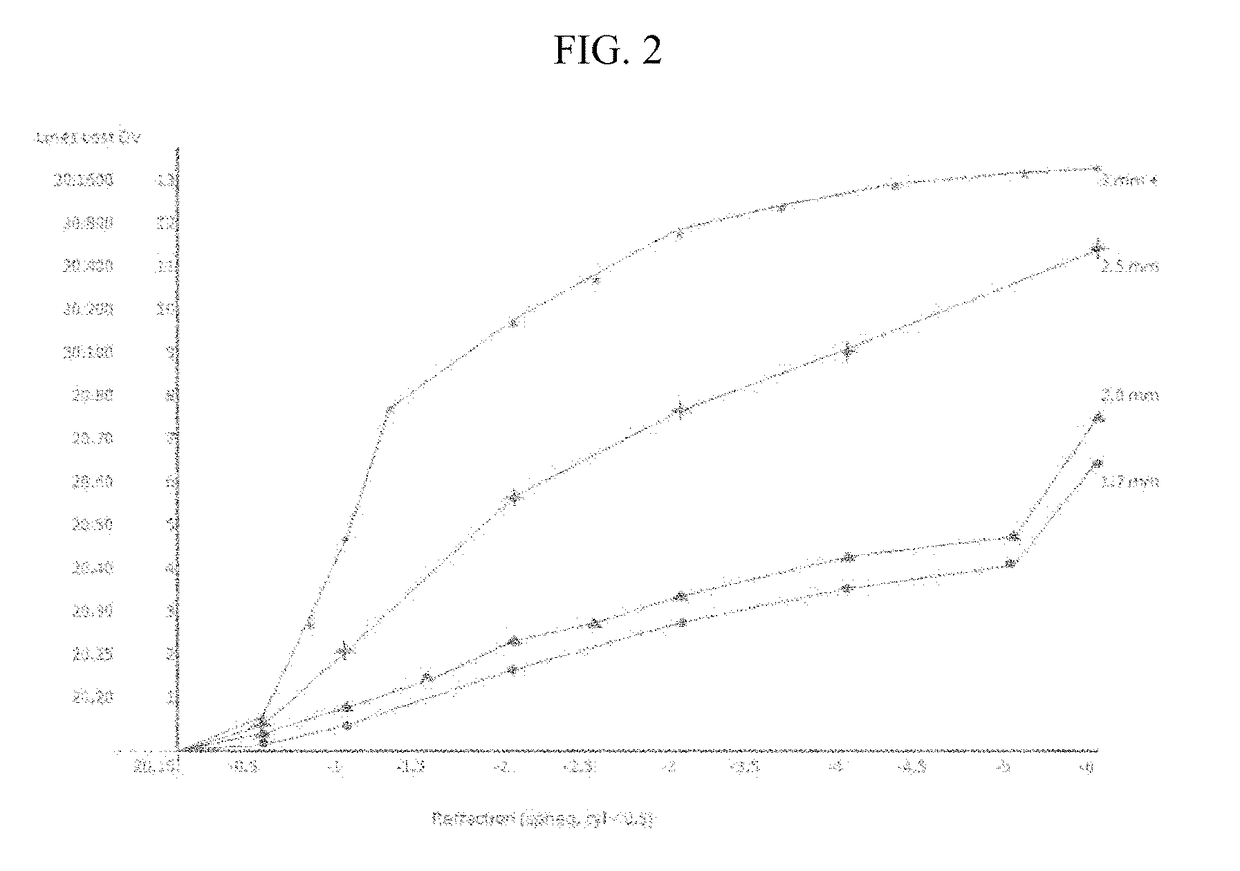
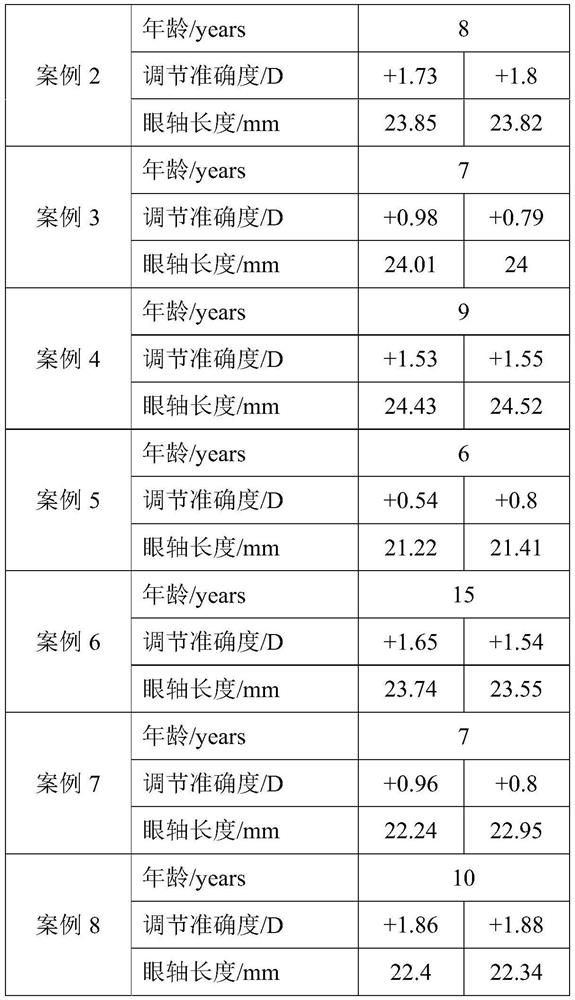
A method for predicting the change of spherical equivalent power before and after cycloplegia
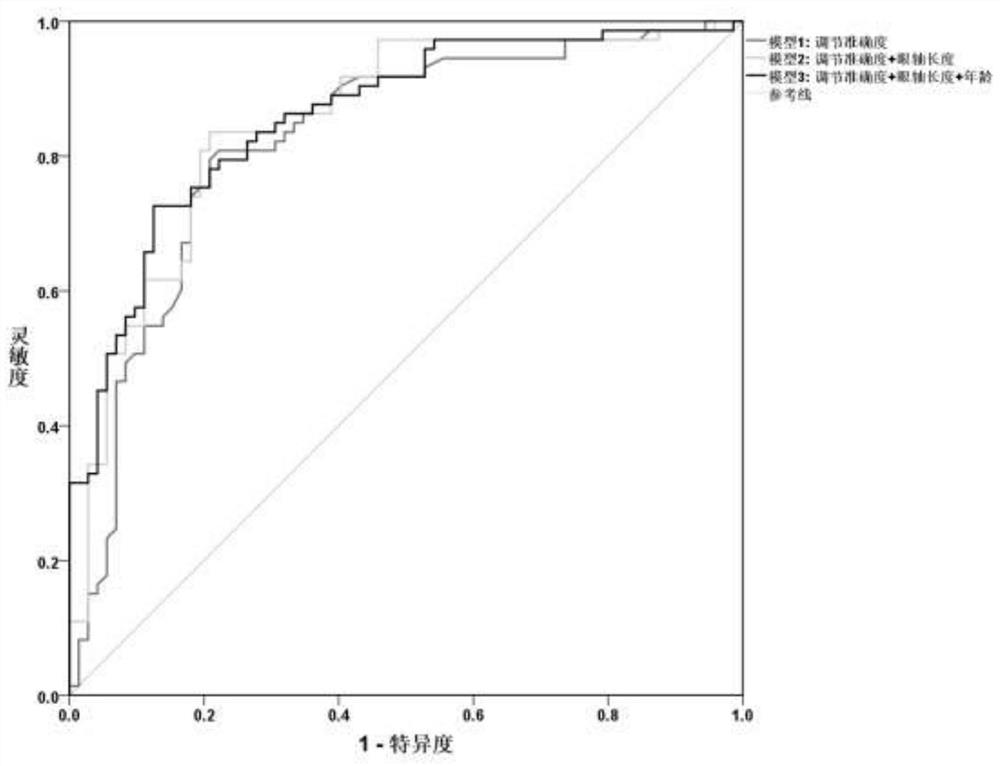
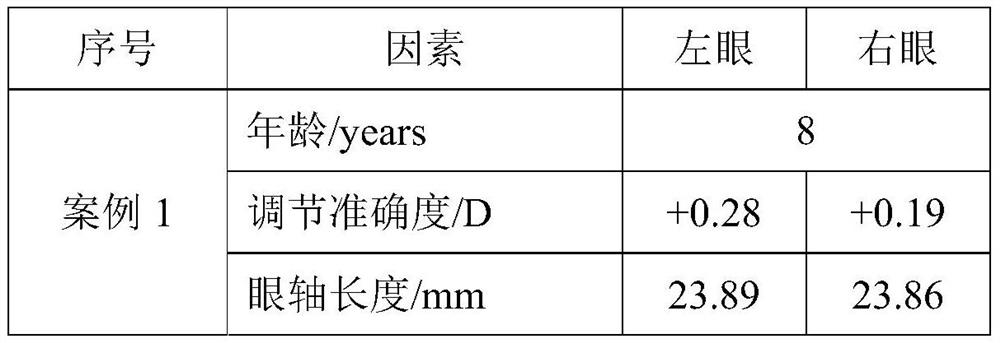
ActiveCN111145899BReduce exposure to related drug side effectsEffective forecastMedical data miningMedical automated diagnosisEye SurgeonCILIARY MUSCLE PARALYSIS
The invention relates to a method for estimating spherical equivalent change degree before and after cycloplegia. The spherical equivalent change degree is estimated by measuring and adjusting an accuracy numerical value; in order to further improve the prediction accuracy, the eye axis length and the subject age can be increased to serve as reference factors, and a prediction result is obtained through a calculation model. The beneficial effects of the invention are that the method can achieve the effective estimation of the spherical equivalent change degree before and after cycloplegia; themethod is higher in predictability and can provide a new clinical standard for clinical ophthalmologists to evaluate whether children patients need to use a cycloplegia agent or not, so the diagnosisand treatment efficiency is improved, medical resources are saved, and related drug side effects borne by the patients due to unnecessary cycloplegia are reduced.
Owner:天津医科大学眼科医院
Aceclidine isomers and scalemic mixtures thereof for the treatment of presbyopia
InactiveUS20200061036A1Organic active ingredientsSenses disorderActive agentAdrenergic receptor agonists
The invention provides compositions containing aceclidine isomers and scalemic mixtures thereof for the treatment of presbyopia. The compositions optionally contain an alpha-adrenergic agonist, a cycloplegic agent, a cryoprotectant, a non-ionic surfactant and / or a viscosity enhancer.
Owner:PRESBYOPIA THERAPIES INC
Compositions and methods for the treatment of presbyopia
InactiveCN108883102AReduce myopia blurImproved distance visionOrganic active ingredientsPharmaceutical non-active ingredientsPolyolPreservative
The invention provides compositions and methods for the treatment of presbyopia and other vision defects. The compositions preferably comprise aceclidine and a polyol and / or a cycloplegic agent. The compositions optionally contain a surfactant, a viscosity enhancer, an osmolarity modifier and a preservative.
Owner:PRESBYOPIA THERAPIES
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com