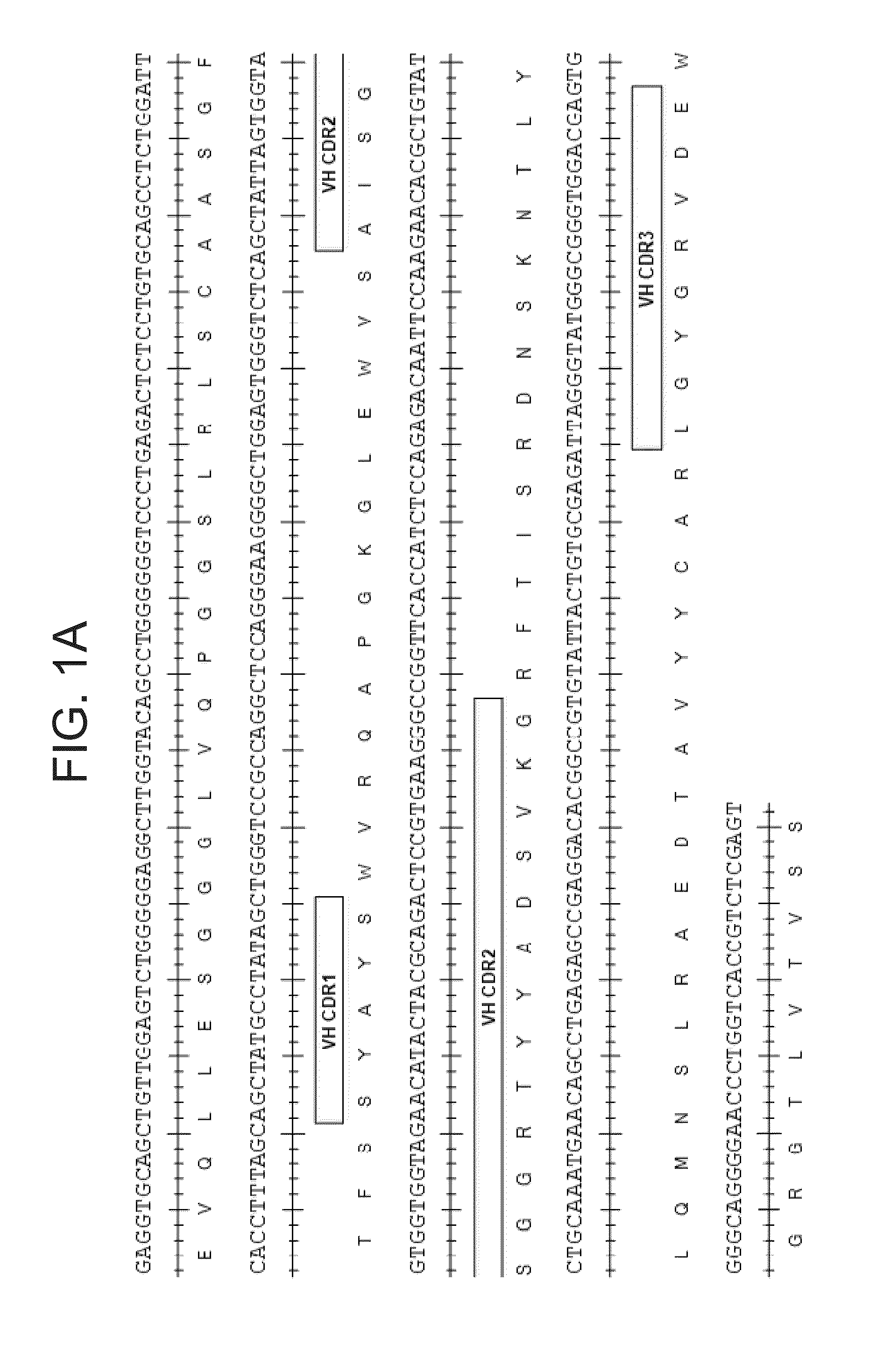
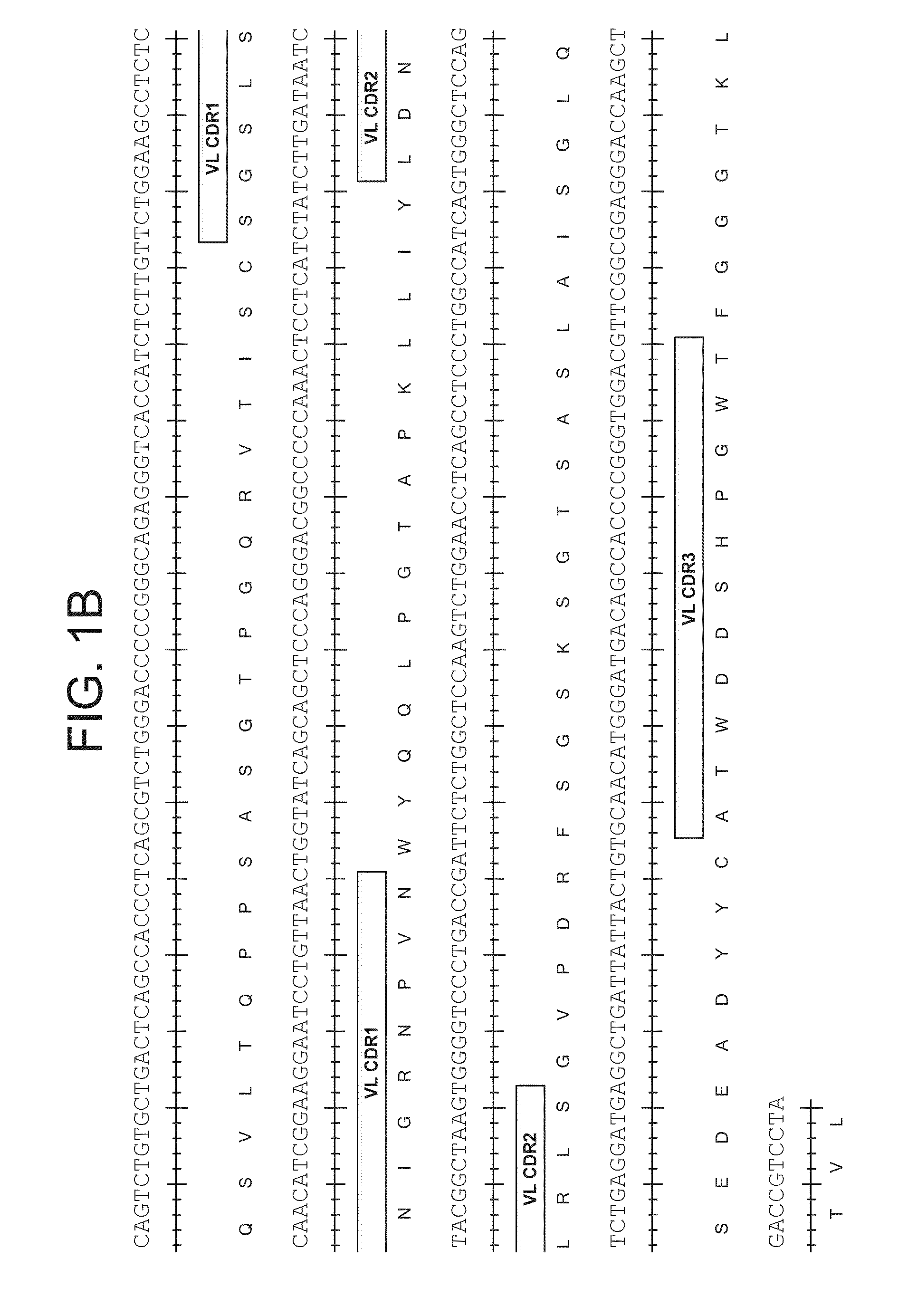
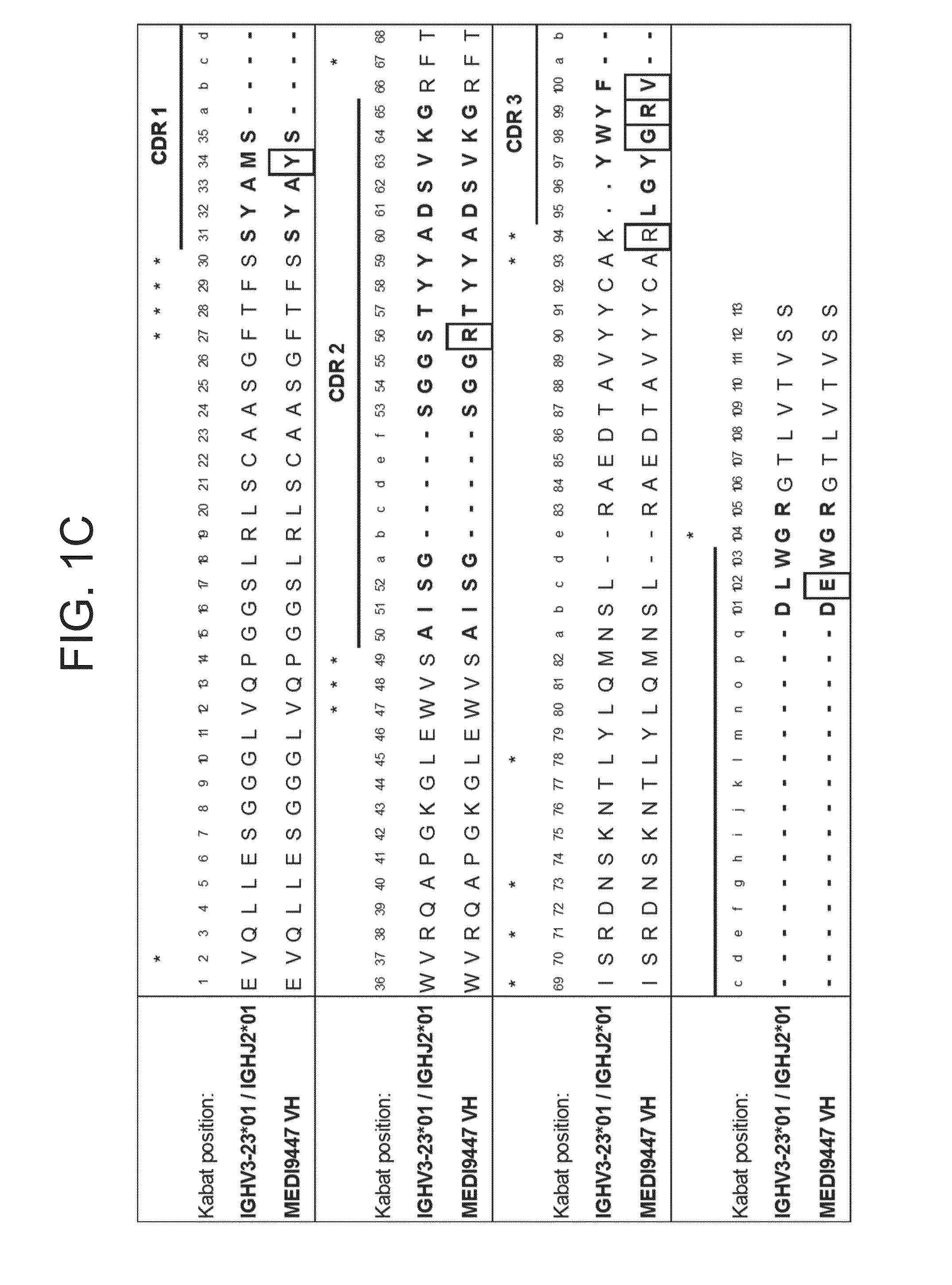
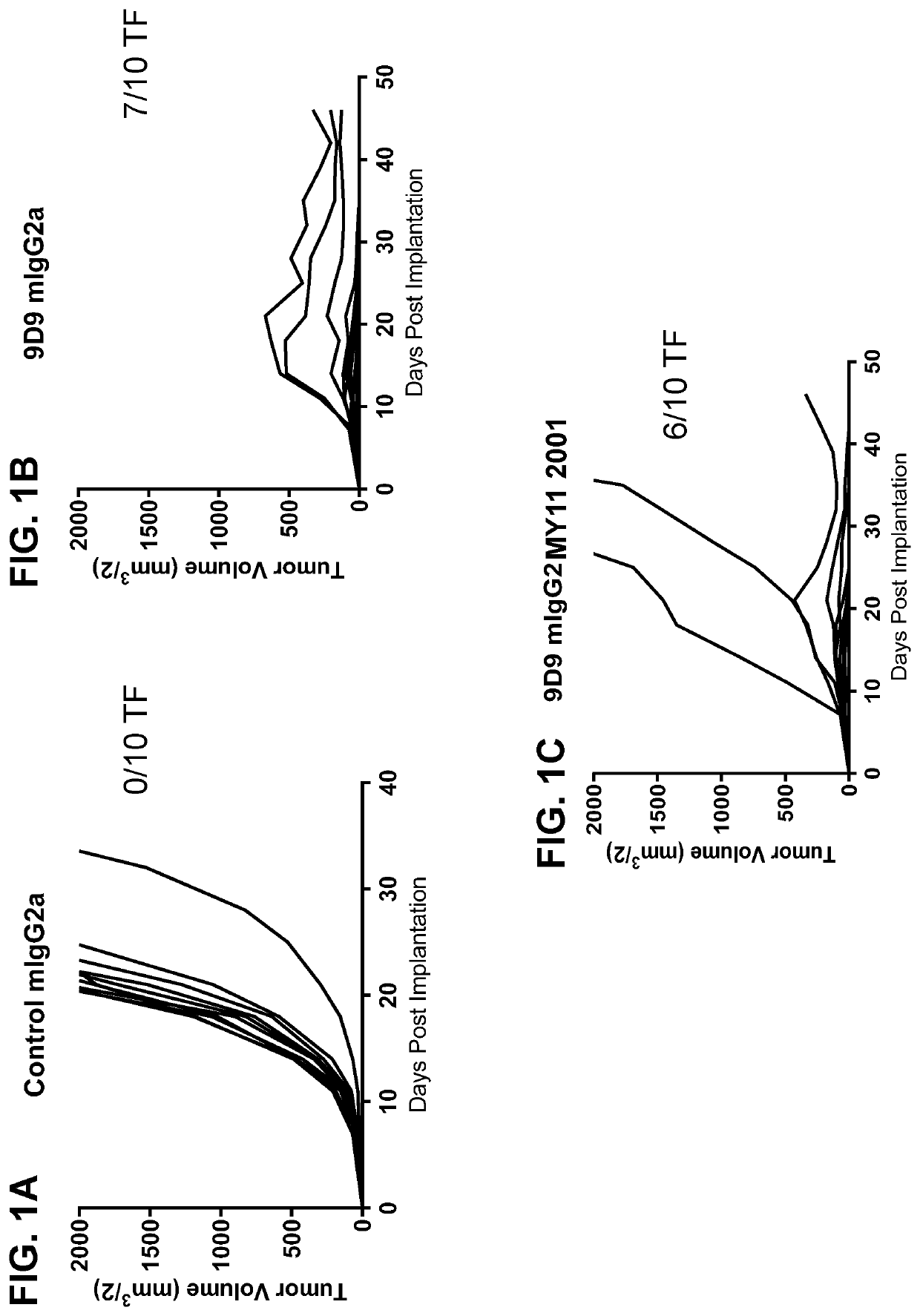
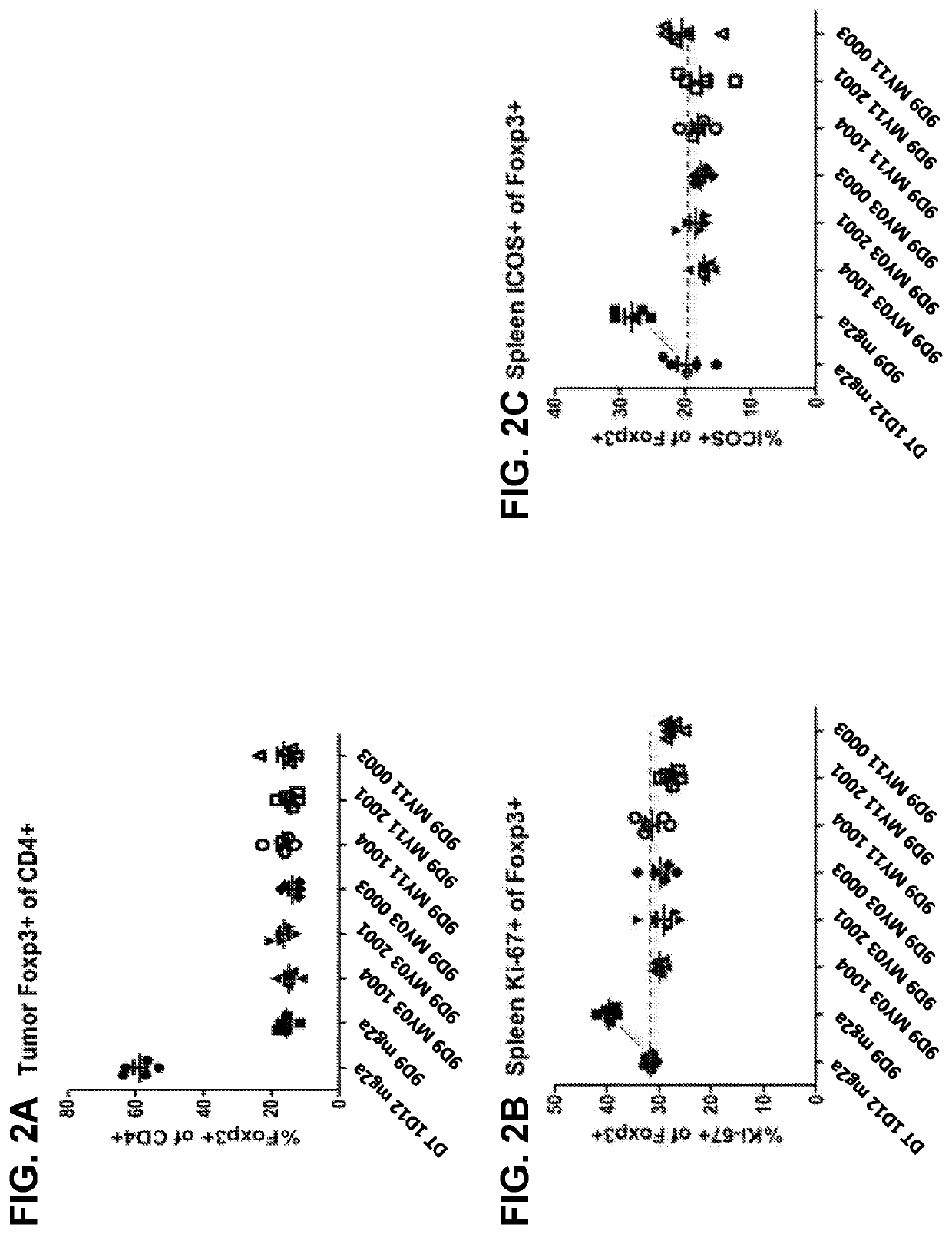
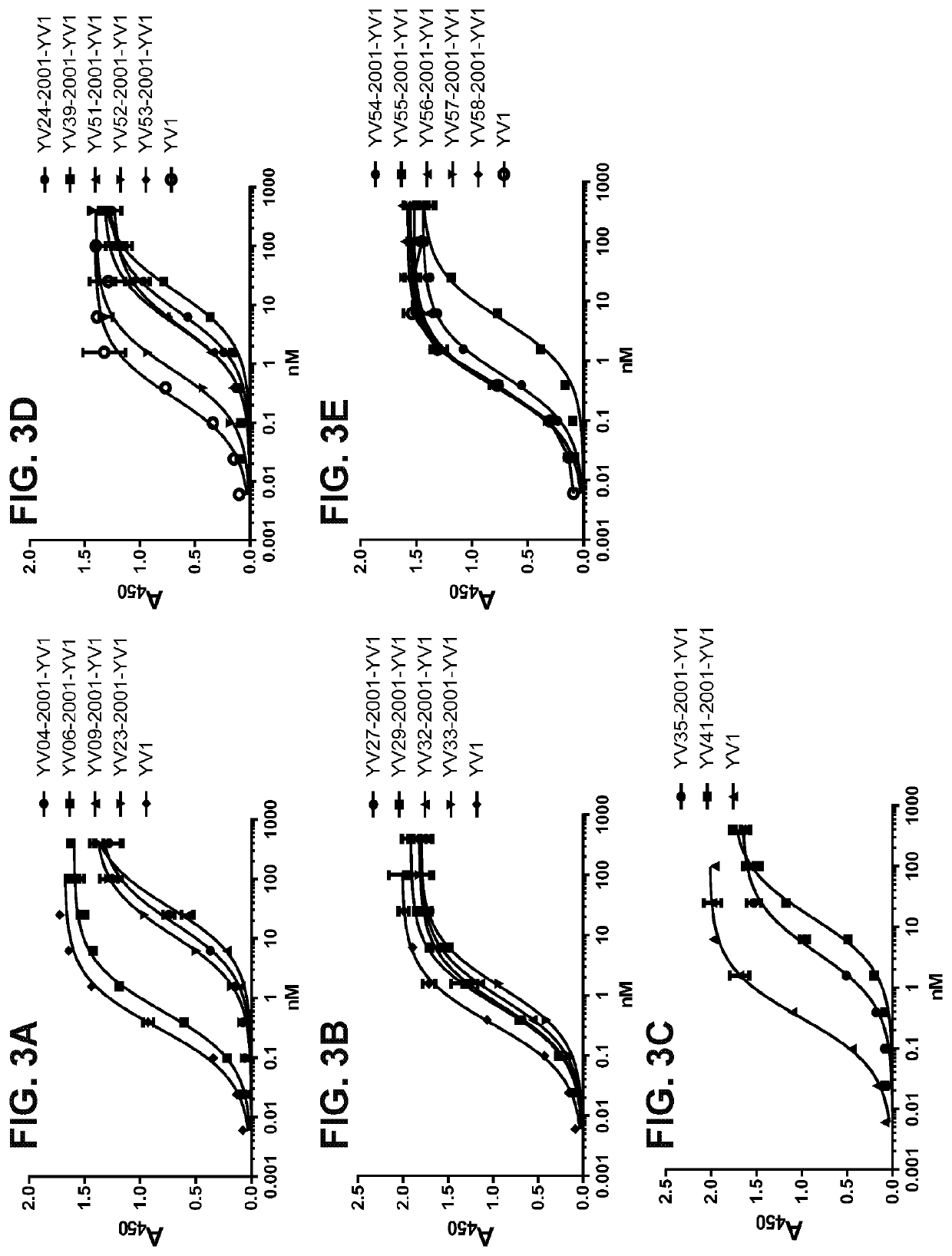
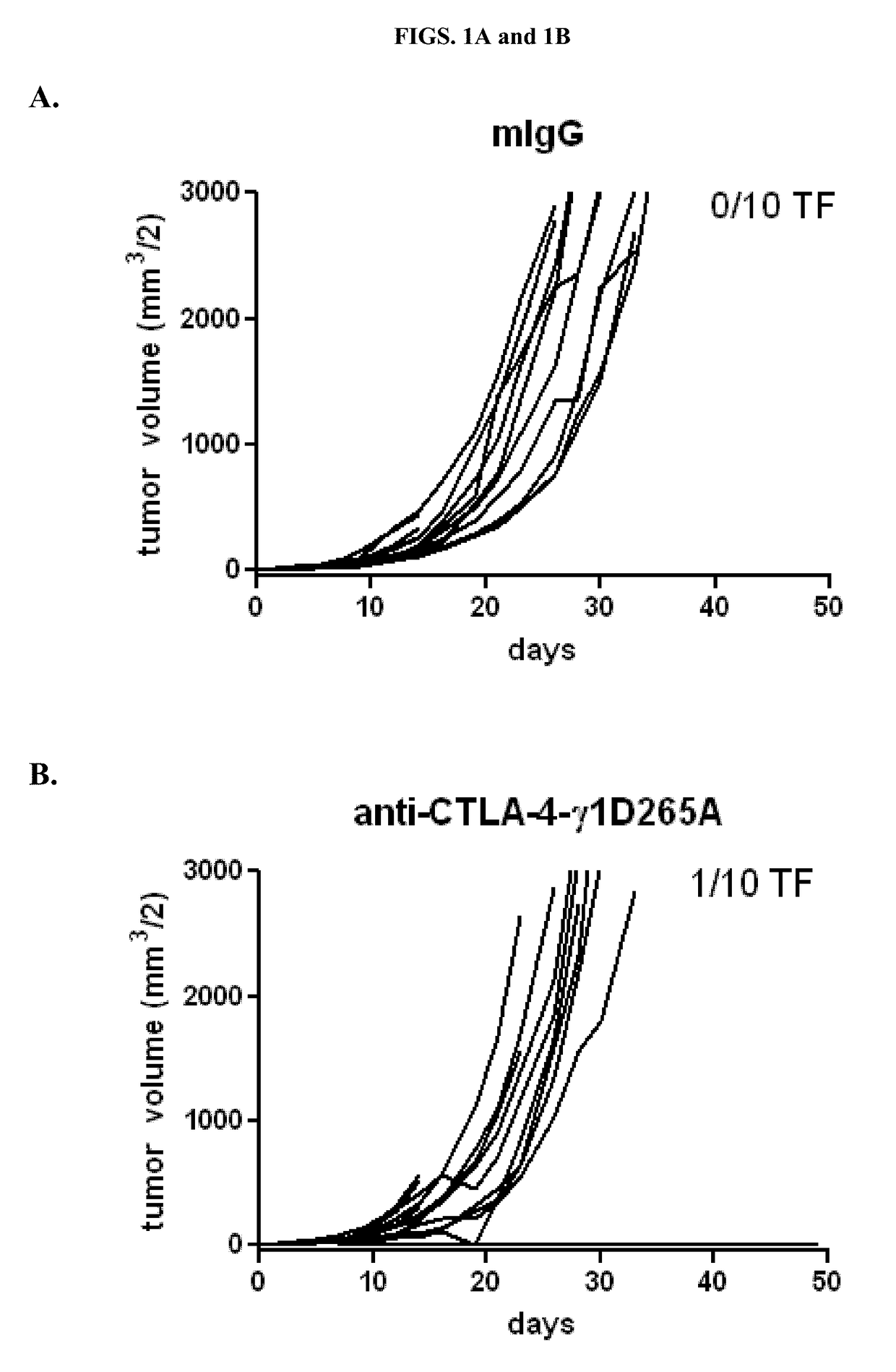
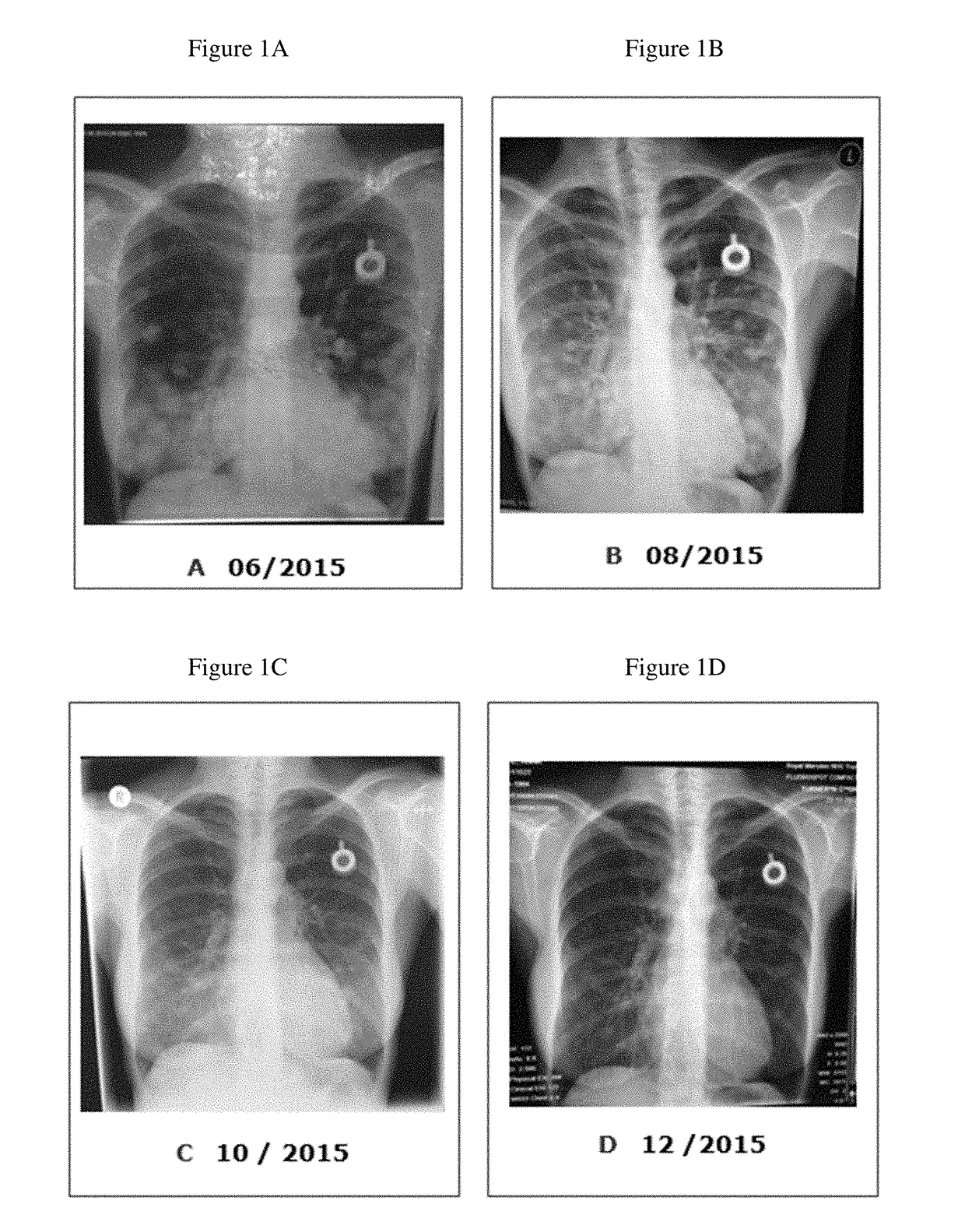
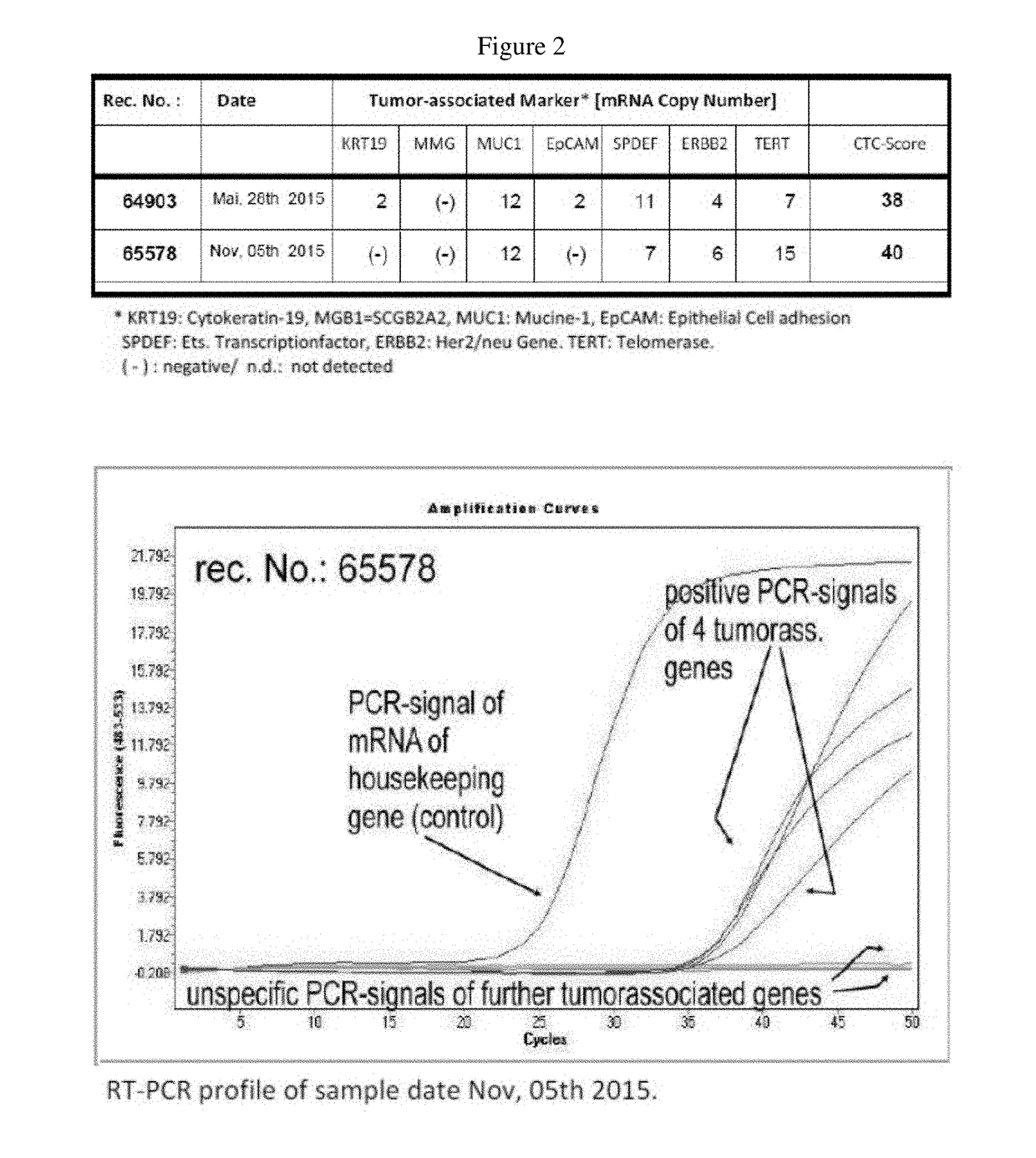
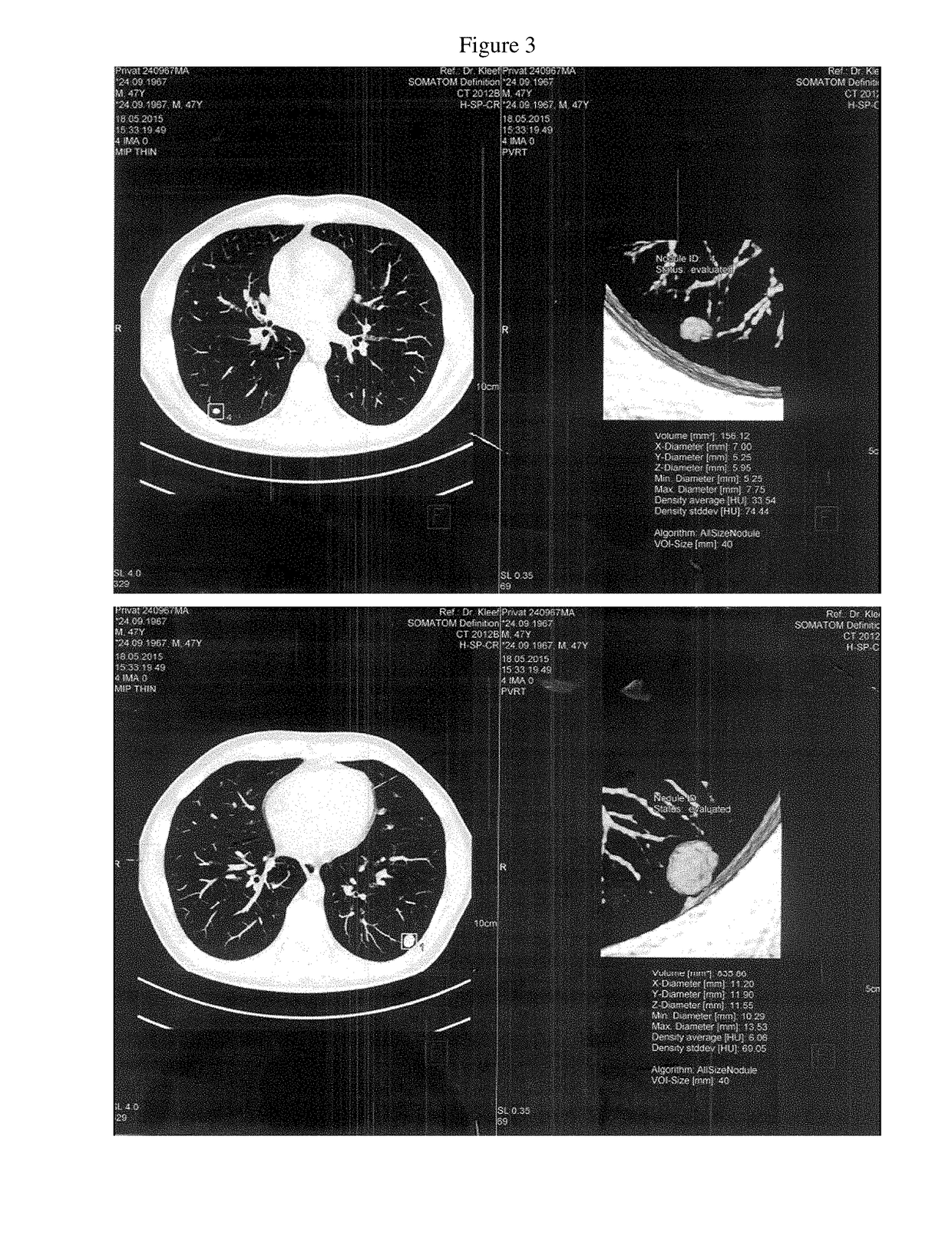
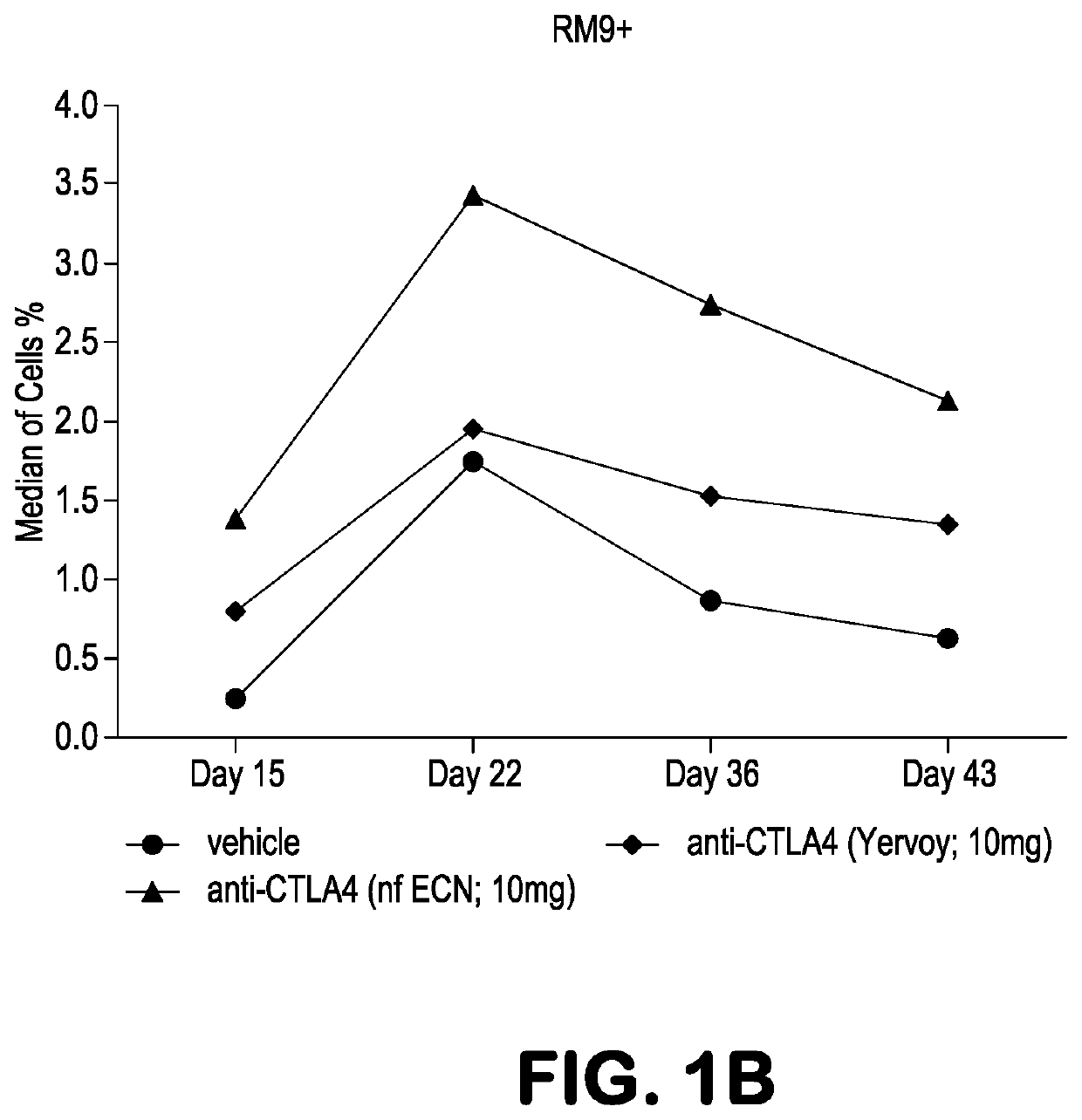
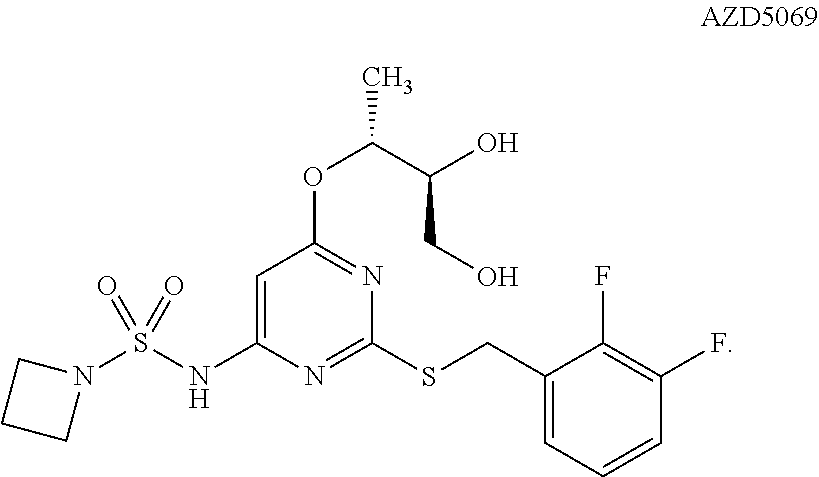
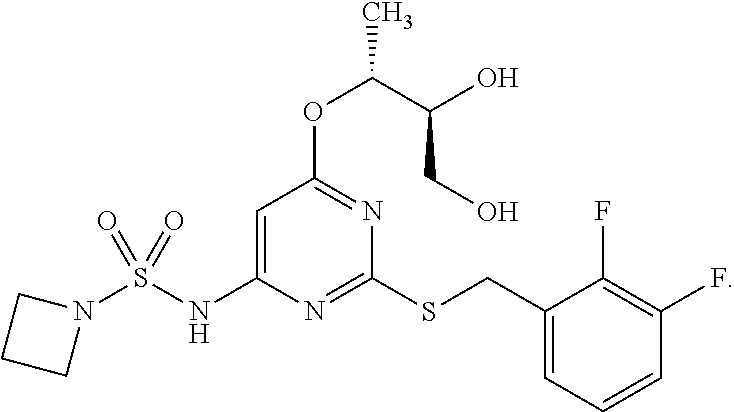
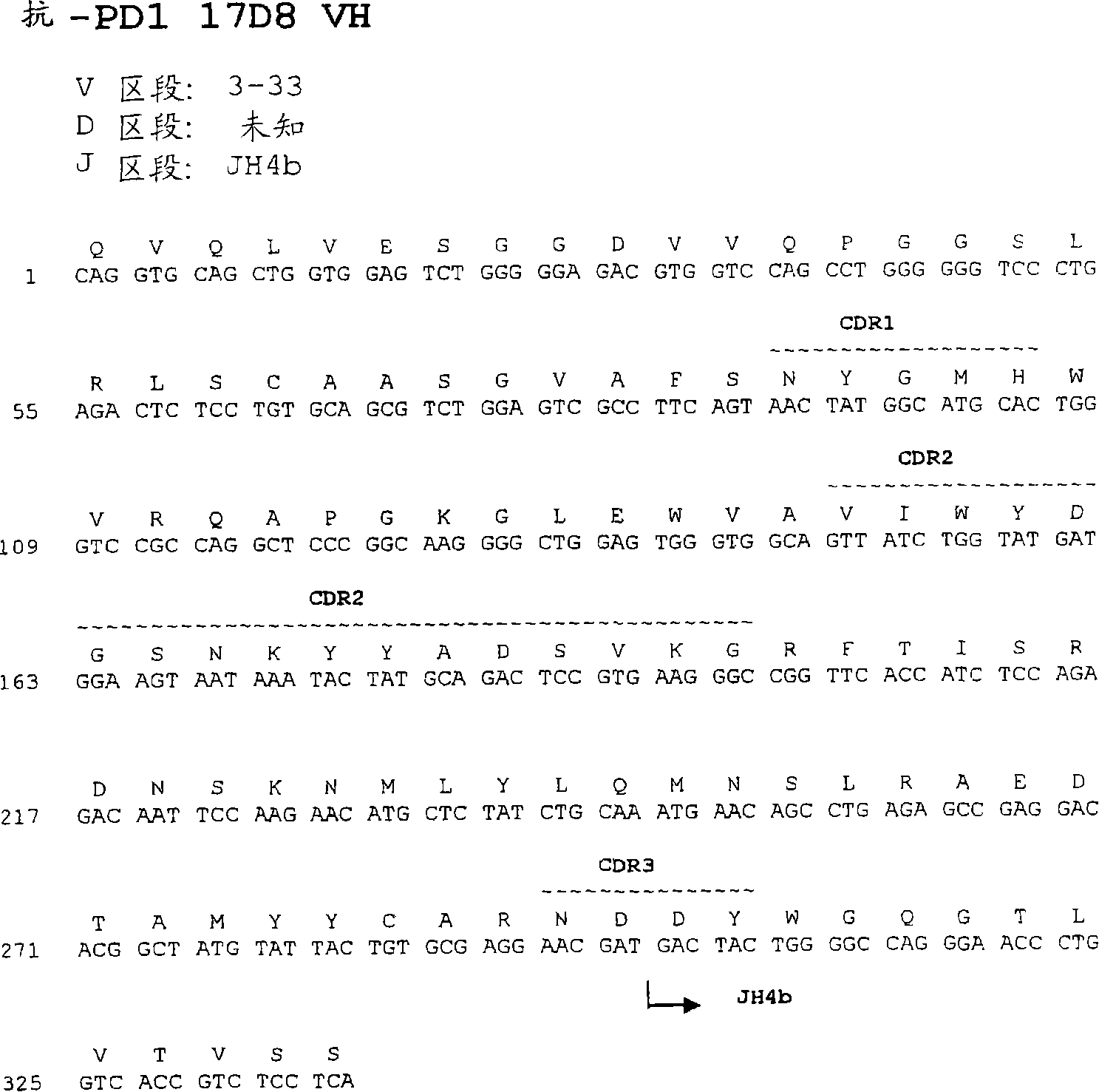Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
53 results about "Anti ctla 4" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
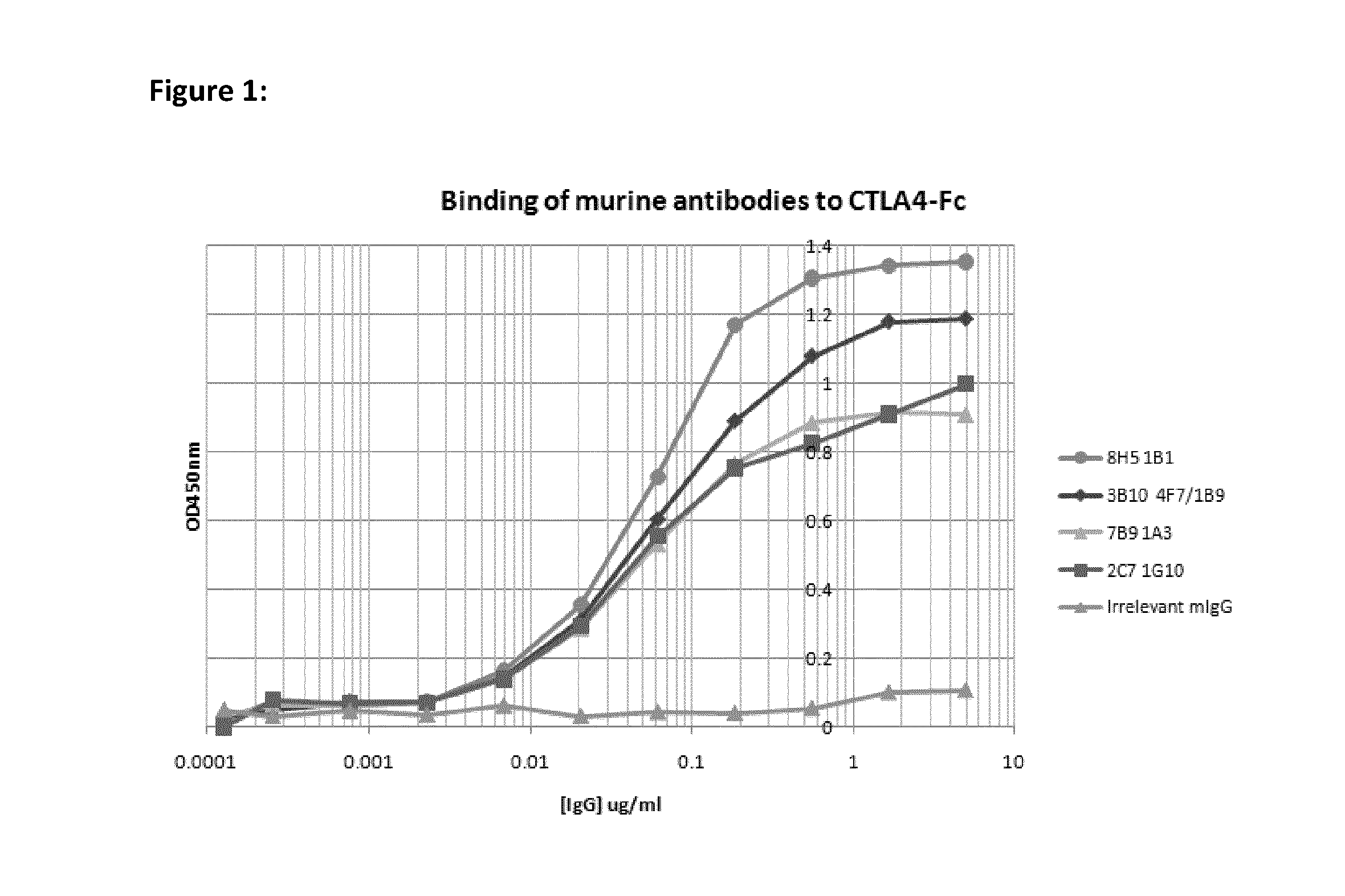
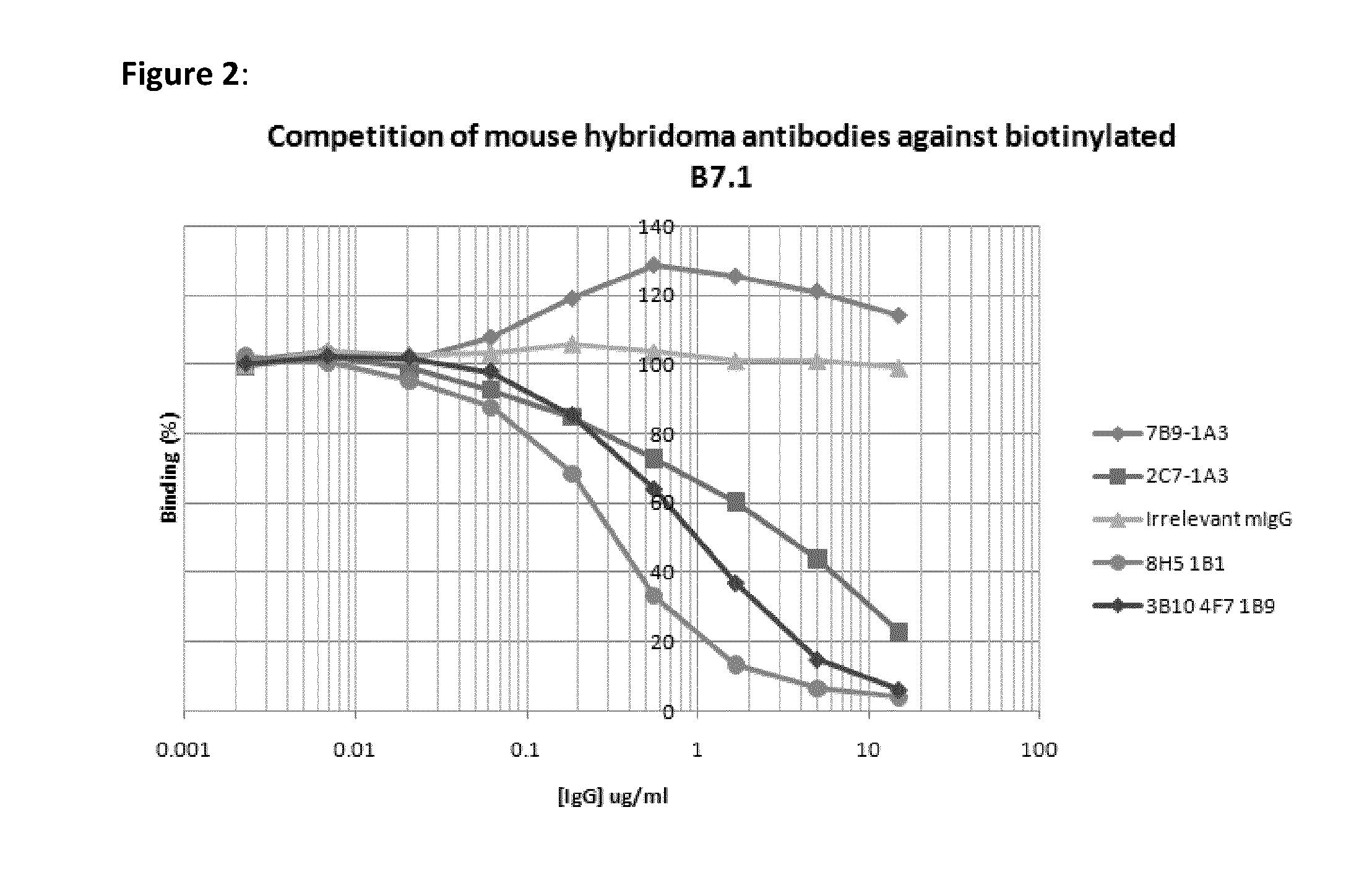
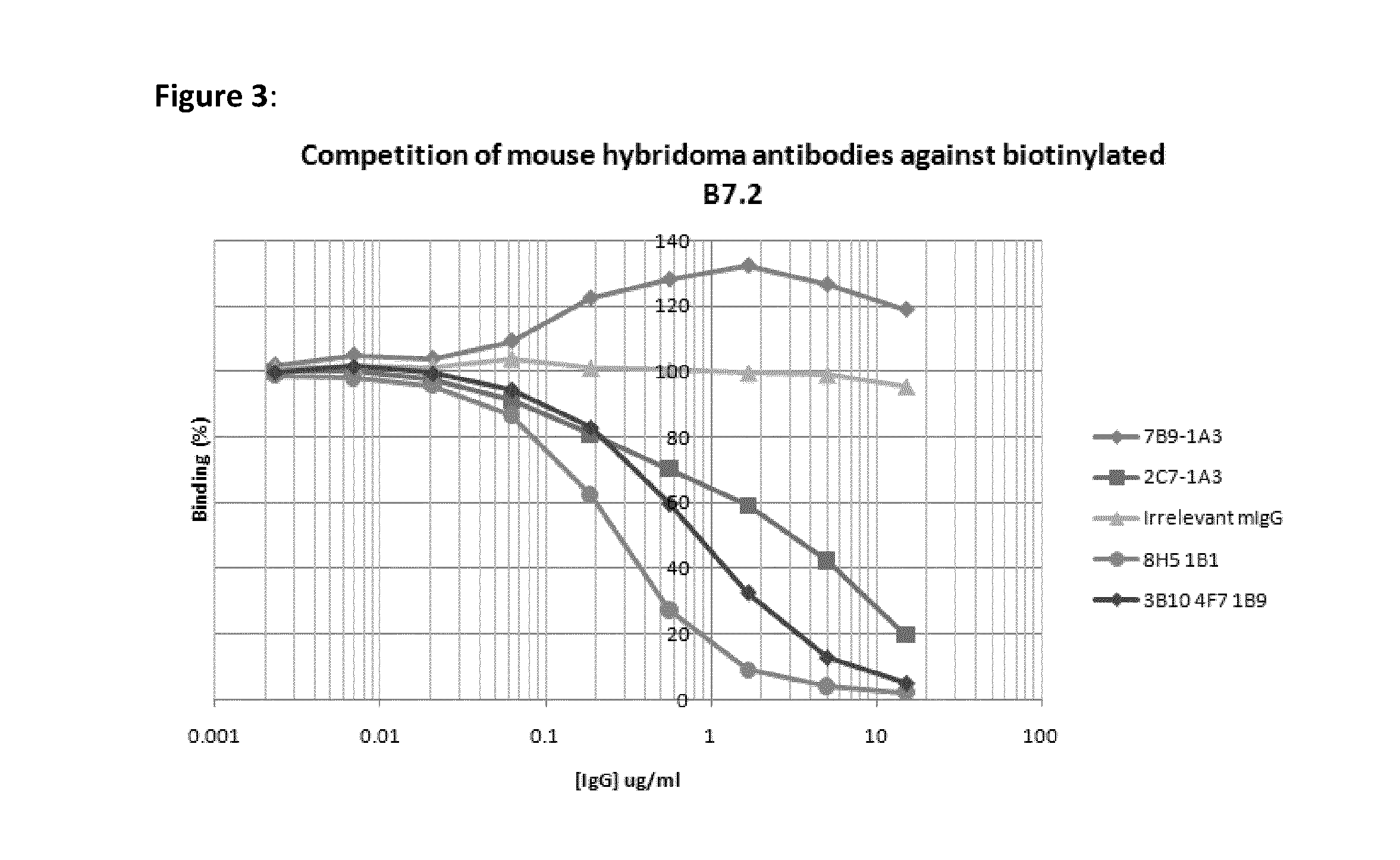
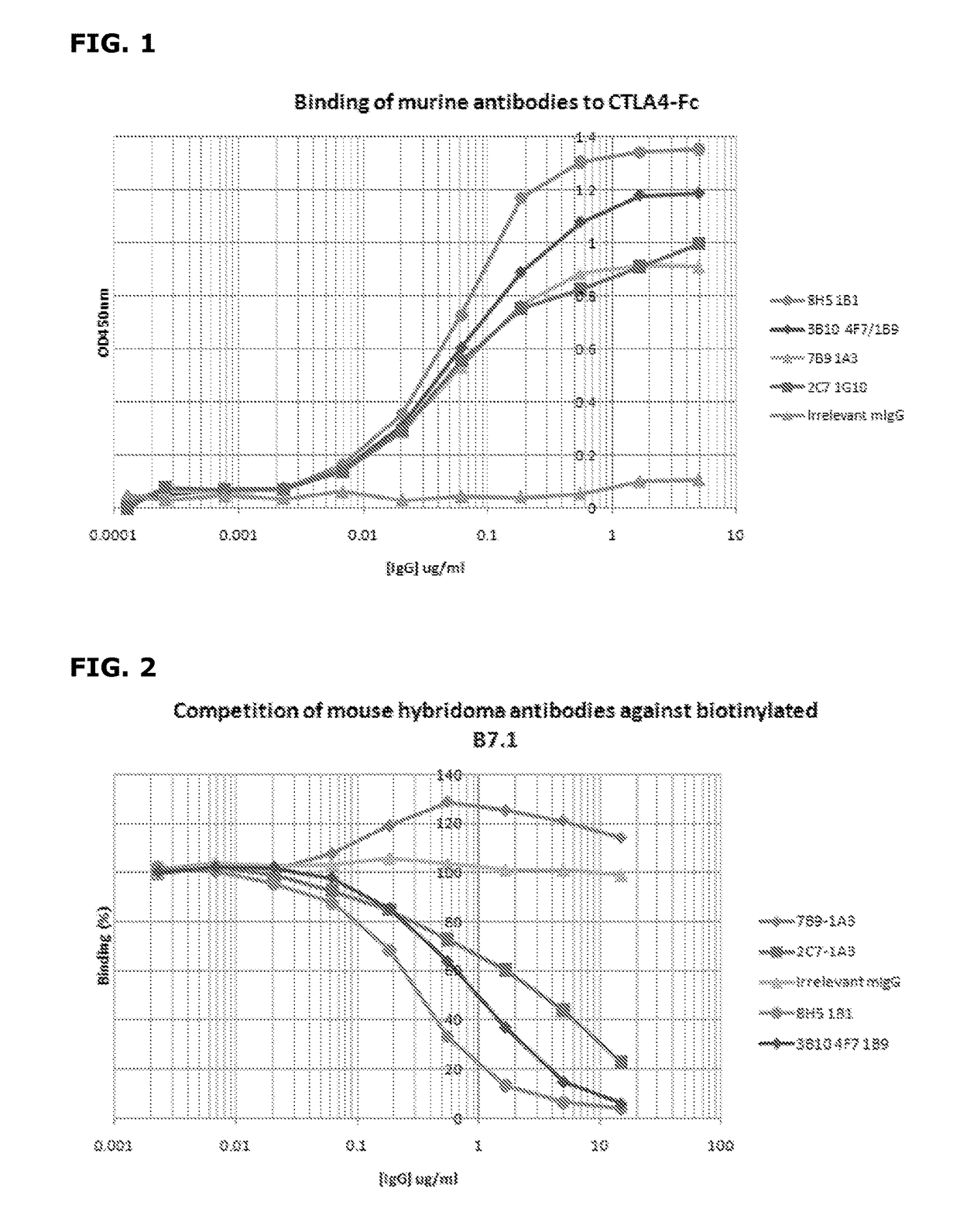
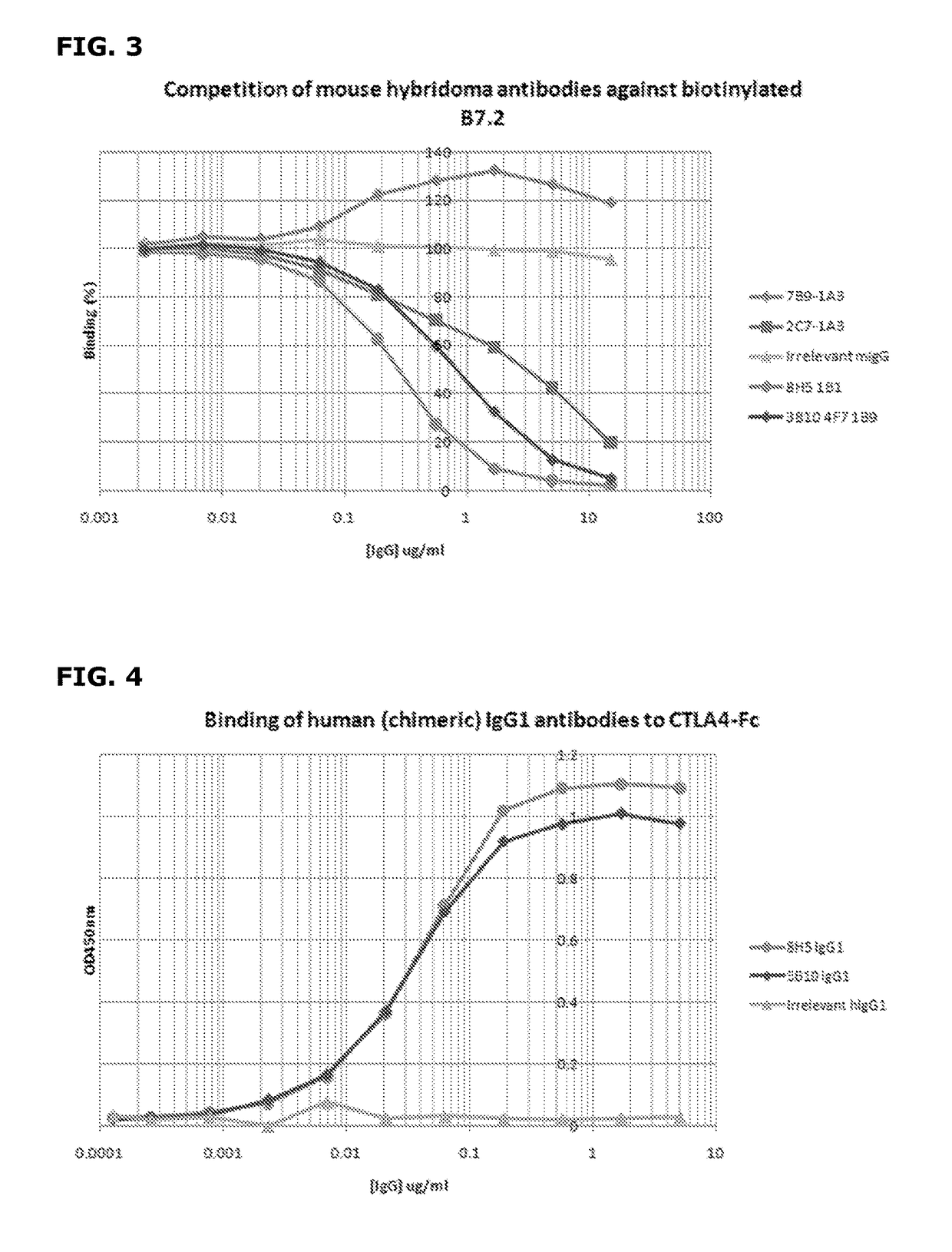
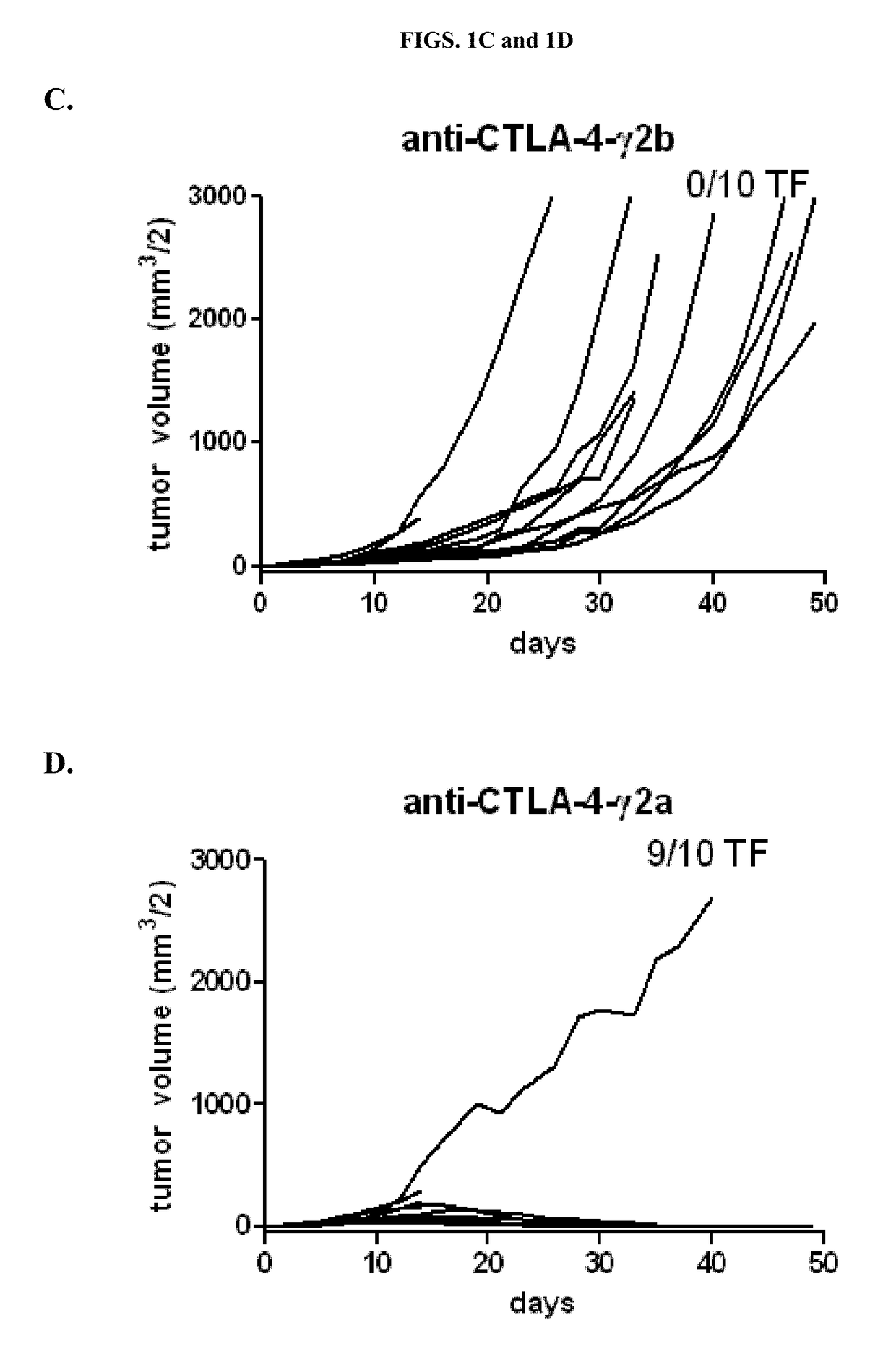
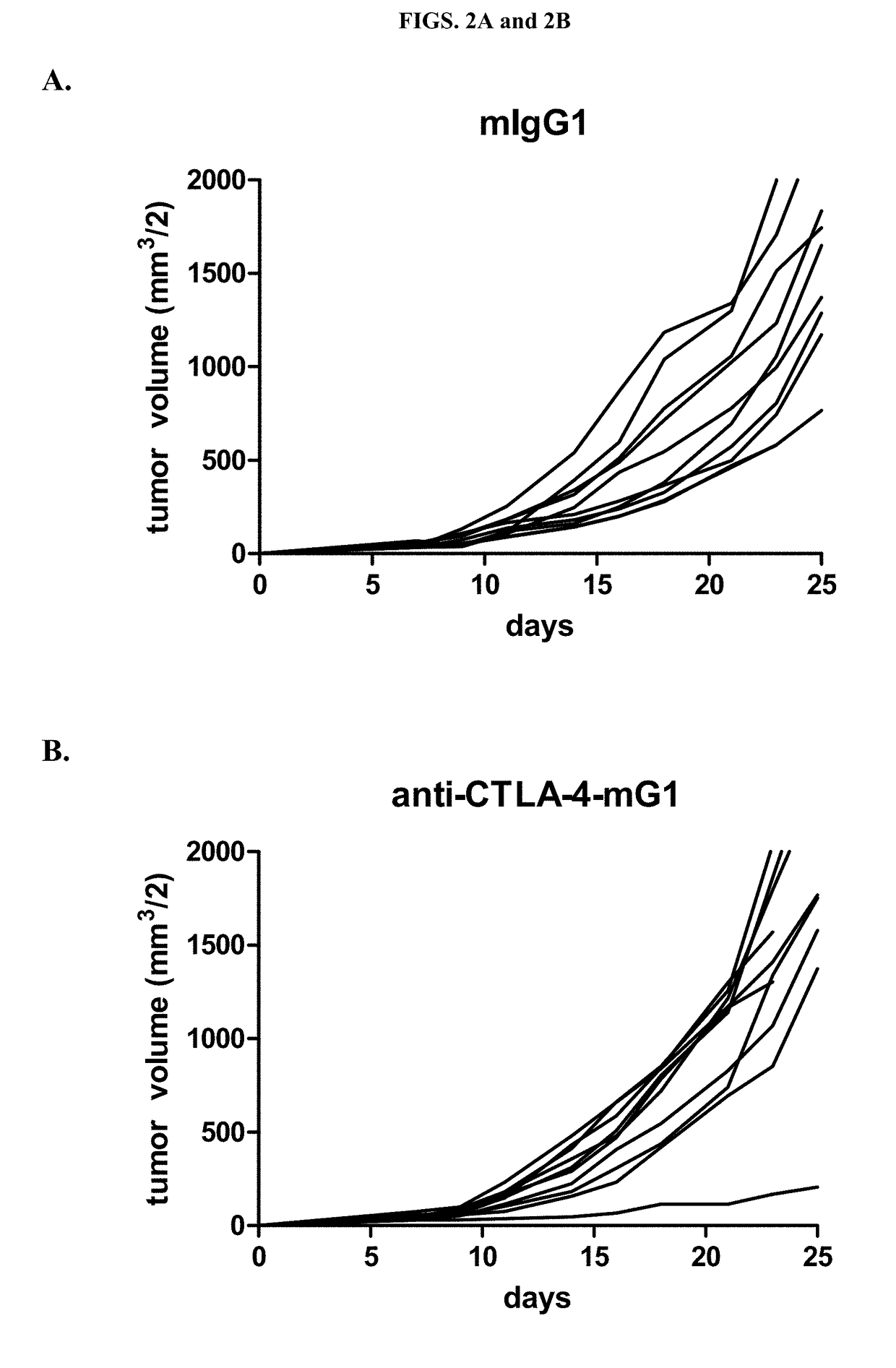
Cytotoxic T lymphocyte-associated antigen (CTLA-4), also known as CD152, is a co-inhibitory molecule that functions to regulate T cell activation. Antibodies that block the interaction of CTLA-4 with its ligands B7.1 and B7.2 can enhance immune responses, including anti-tumor immunity.
Cancer immunotherapy by disrupting pd-1/pd-l1 signaling
ActiveUS20150125463A1Reliable responseReduces and suppresses signalingImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsAntigenTissue sample
The disclosure provides a method for immunotherapy of a cancer patient, comprises administering to the patient an Ab that inhibits signaling from the PD-1 / PD-L1 signaling pathway, or a combination of such Ab and an anti-CTLA-4 Ab. This disclosure also provides a method for immunotherapy of a cancer patient comprising selecting a patient who is a suitable candidate for immunotherapy based on an assessment that the proportion of cells in a test tissue sample from the patient that express PD-L1 on the cell surface exceeds a predetermined threshold level, and administering an anti-PD-1 Ab to the selected subject. The disclosure additionally provides rabbit mAbs that bind specifically to a cell surface-expressed PD-L1 antigen in a FFPE tissue sample, and an automated IHC method for assessing cell surface expression in FFPE tissues using the provided anti-PD-L1 Abs.
Owner:BRISTOL MYERS SQUIBB CO
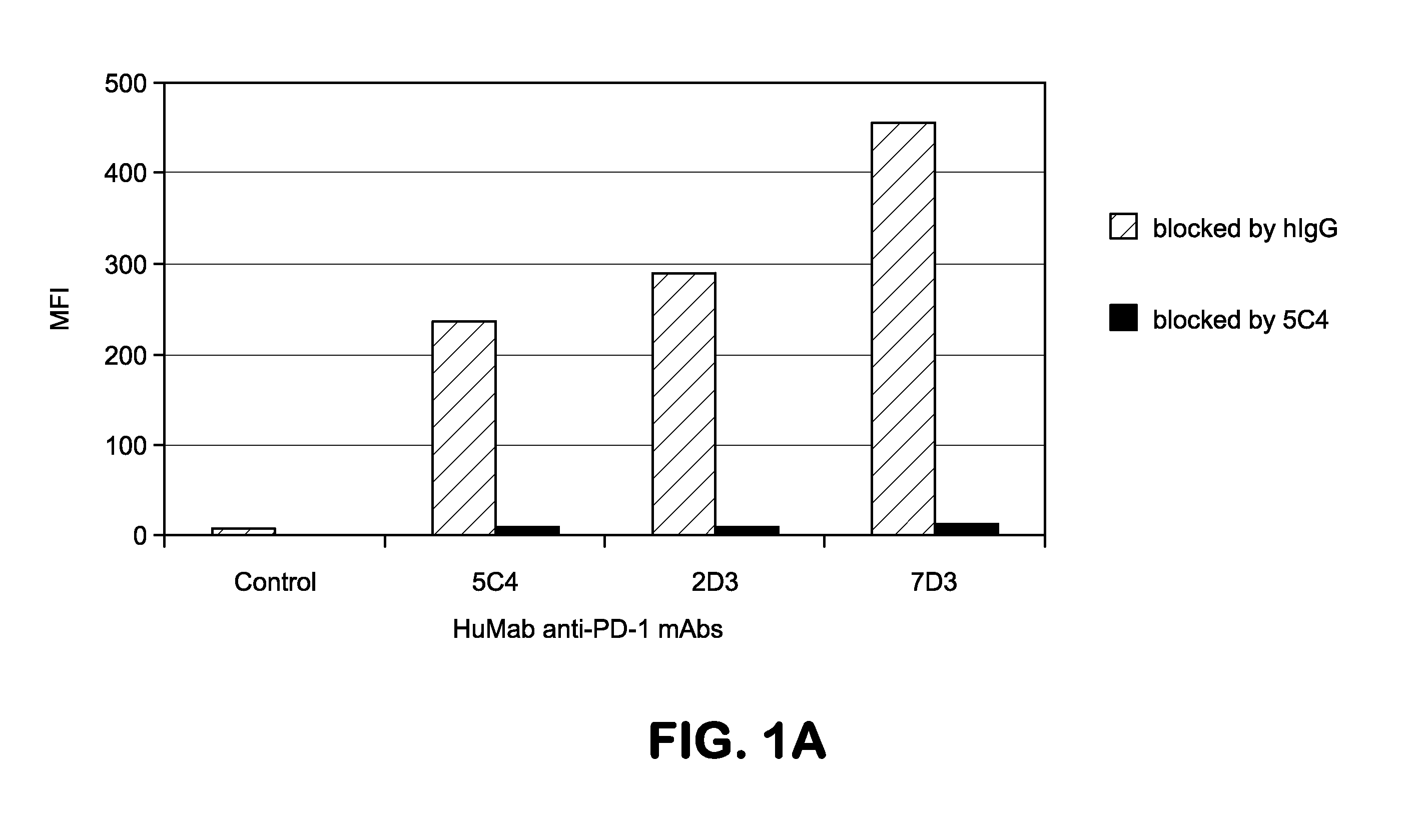
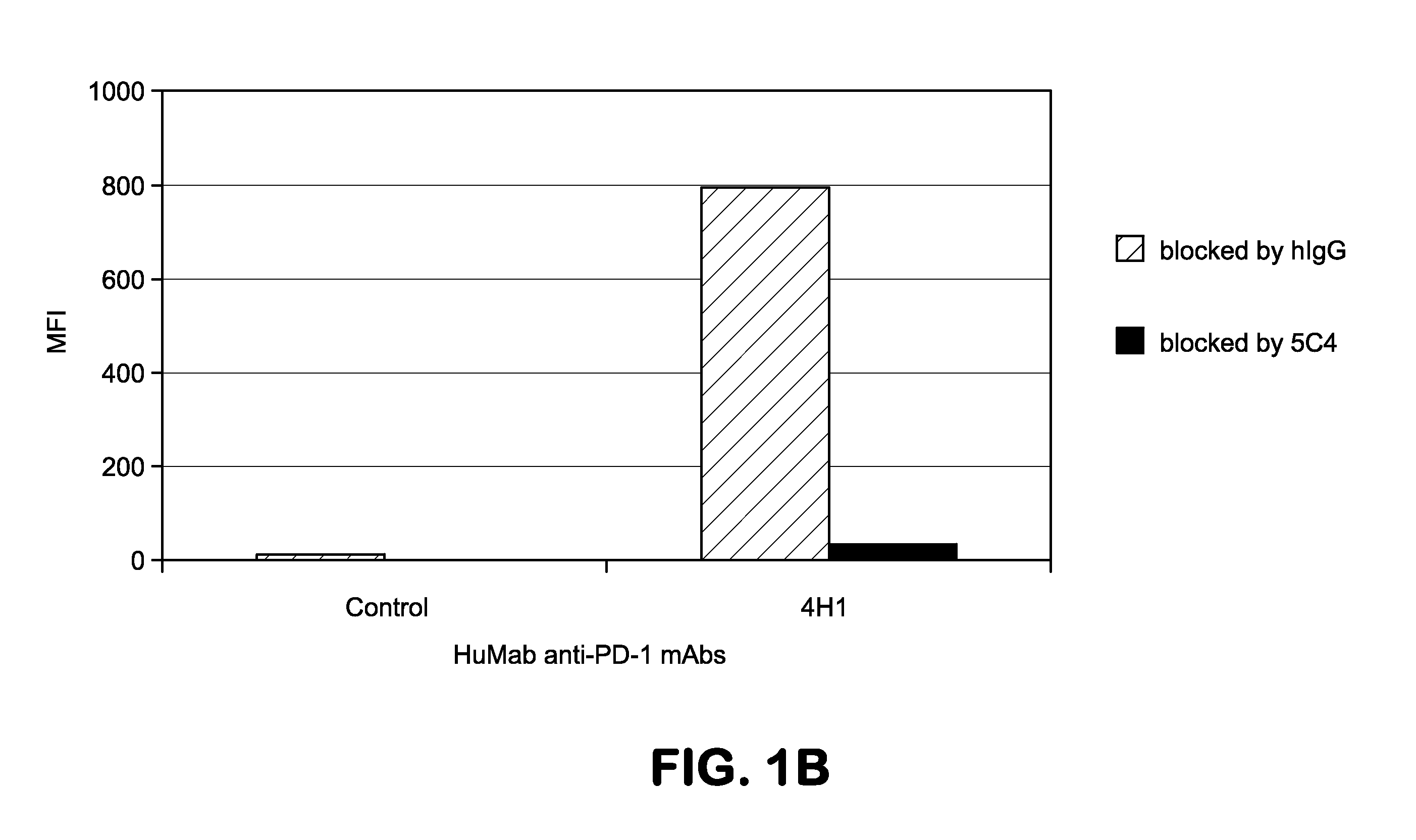
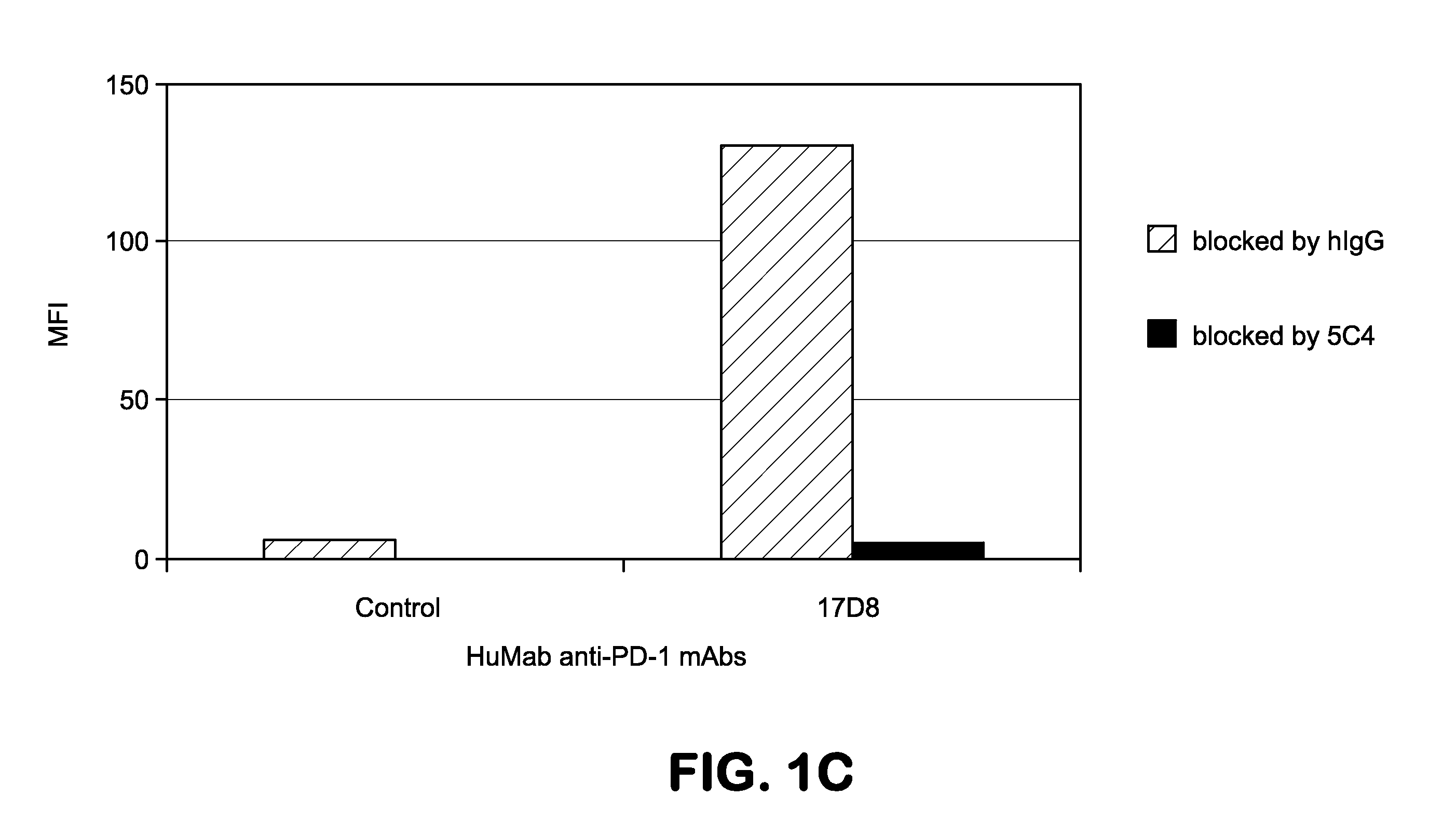
Human monoclonal antibodies to programmed death 1 (PD-1) and methods for treating cancer using anti-PD-1 antibodies alone or in combination with other immunotherapeutics
ActiveCN101213297AMicrobiological testing/measurementImmunoglobulins against cell receptors/antigens/surface-determinantsImmunotherapeutic agentProgrammed death
The present invention provides isolated monoclonal antibodies, particularly human monoclonal antibodies, that specifically bind to PD-1 with high affinity. Nucleic acid molecules encoding the antibodies of the invention, expression vectors, host cells and methods for expressing the antibodies of the invention are also provided. Immunoconjugates, bispecific molecules and pharmaceutical compositions comprising the antibodies of the invention are also provided. The invention also provides methods for detecting PD-1, as well as methods for treating various diseases, including cancer and infectious diseases, using anti-PD-1 antibodies. The present invention further provides methods for using a combination immunotherapy, such as the combination of anti-CTLA-4 and anti-PD-1 antibodies, to treat hyperproliferative disease, such as cancer. The invention also provides methods for altering adverse events related to treatment with such antibodies individually.
Owner:ONO PHARMA CO LTD +1
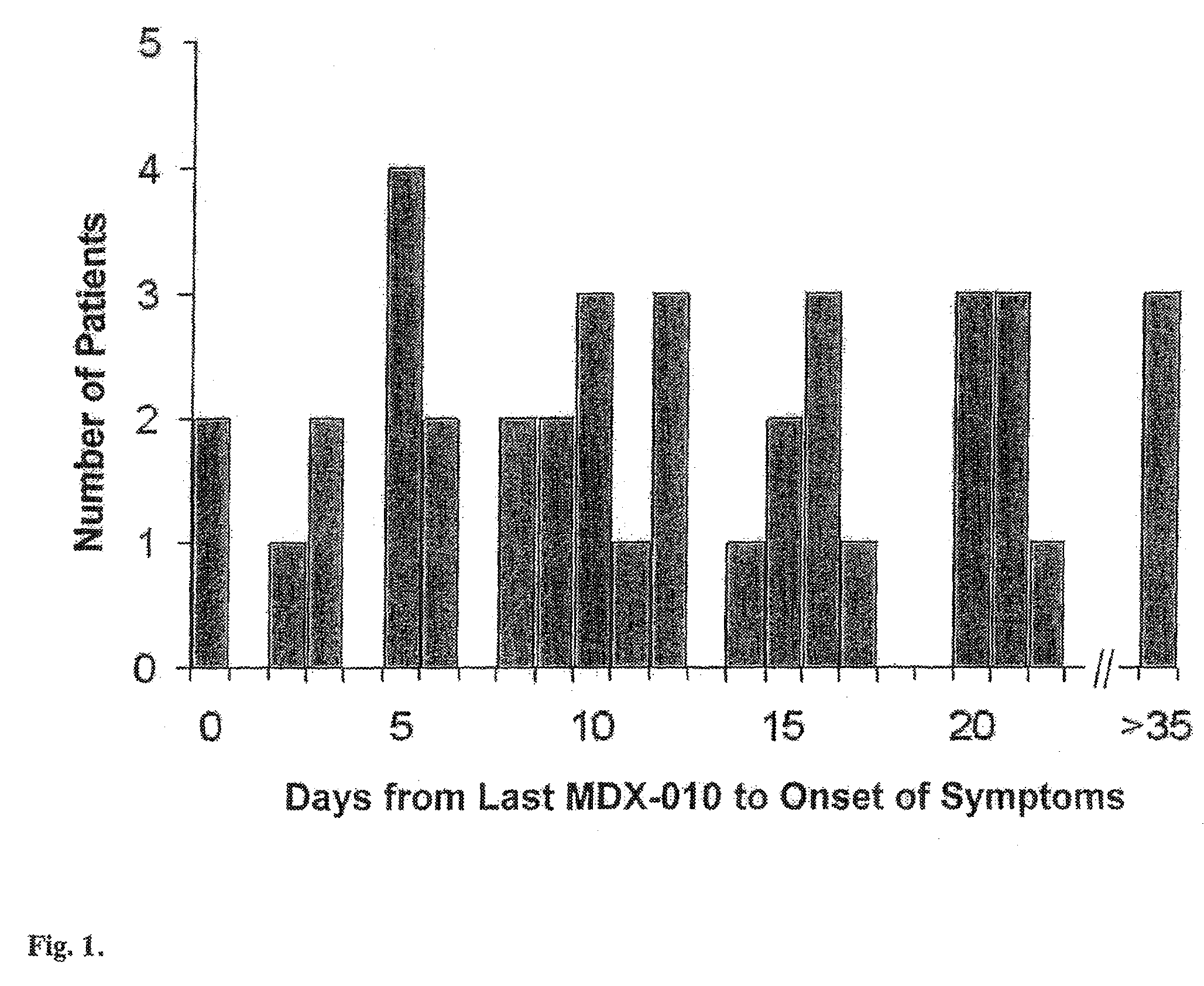
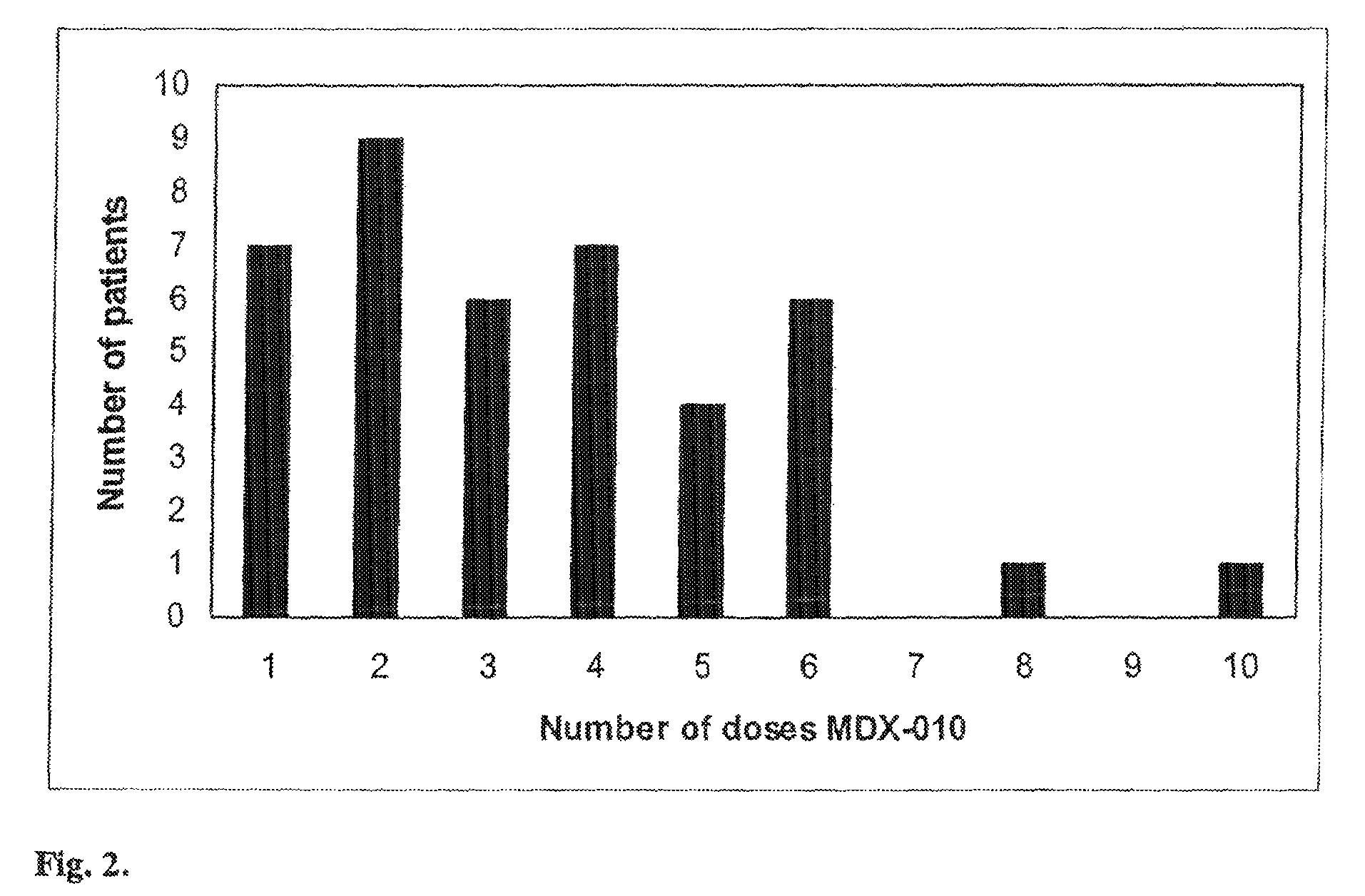
Prophylaxis and treatment of enterocolitis associated with Anti-ctla-4 antibody therapy
InactiveUS20070243184A1Reduce inflammationReduce morbidityOrganic active ingredientsGenetic material ingredientsEnterocolitisImmunotherapy
The present invention provides methods for reducing the incidence of adverse events related to immunotherapy. More specifically, the present invention provides methods for reducing the incidence of enterocolitis associated with anti-CTLA-4 antibody immunotherapy.
Owner:CEDARS SINAI MEDICAL CENT +2
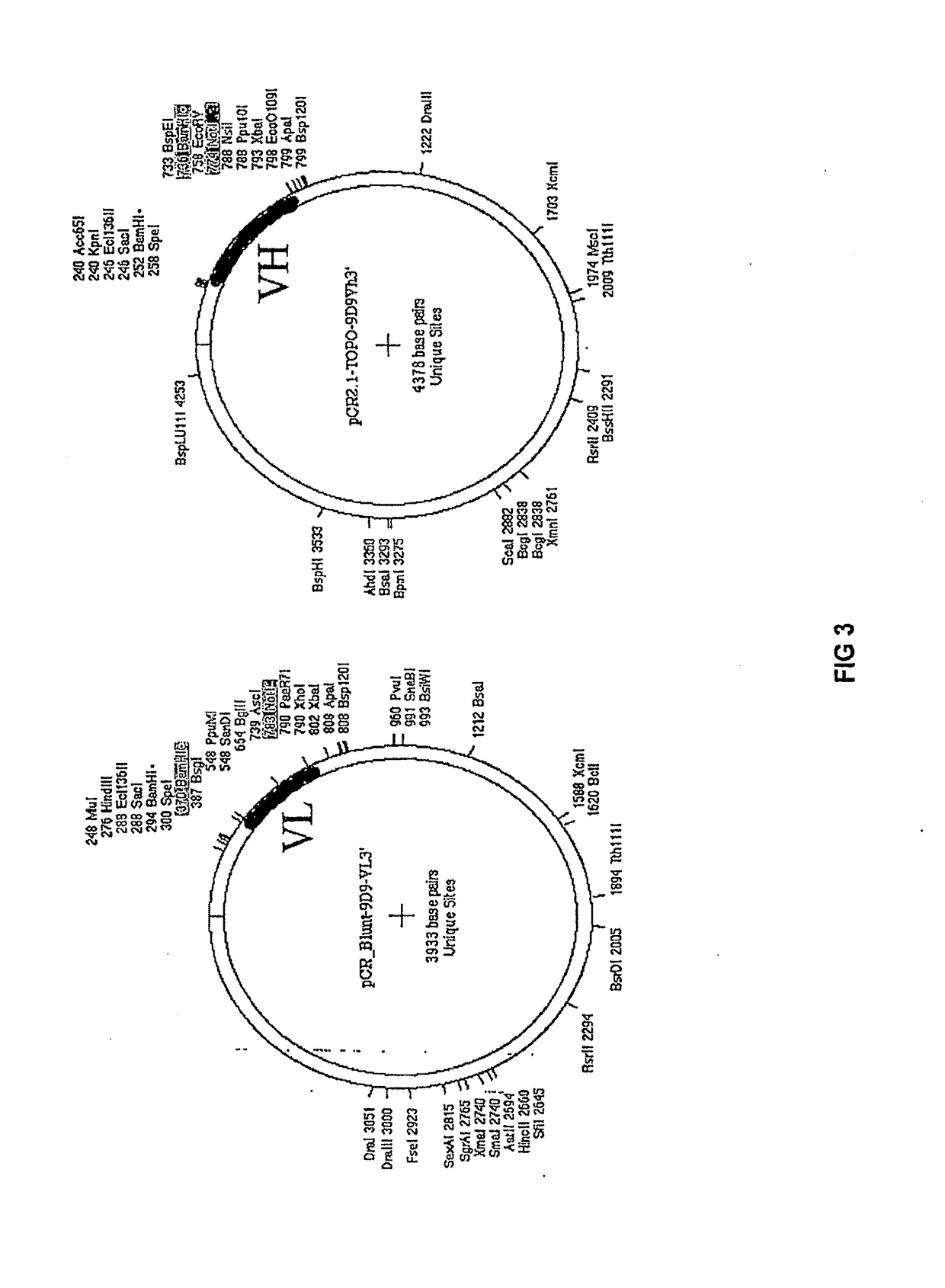
Anti-CTLA-4 antibodies
ActiveUS9758583B2Recovery functionPolypeptide with localisation/targeting motifAntibody mimetics/scaffoldsAntibodyAnti ctla 4
Owner:AGENCY FOR SCI TECH & RES
Humanised anti ctla-4 antibodies
ActiveUS20140105914A1Inducing, augmenting or prolonging an immune response to an antigen in a patientEnhance immune responseNervous disorderAntipyreticDiseaseSpecific antibody
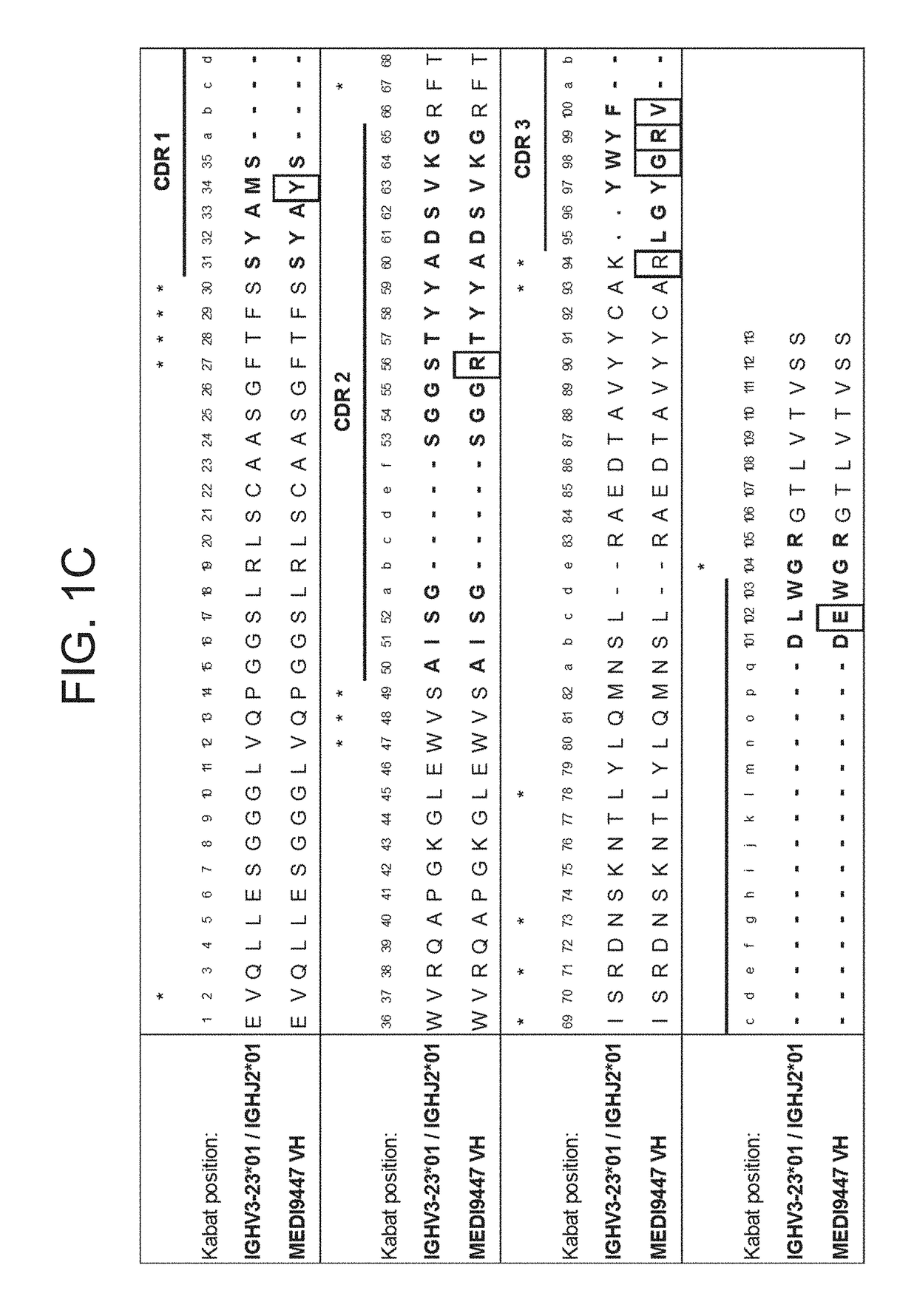
The invention provides an anti-CTLA4 antibody which inhibits the binding of CTLA4 to human B7, in particular, it inhibits binding of CTLA4 to human B7.1 and / or human B7.2. Specific antibodies are provided with specific variable region sequences as well as compositions comprising such antibodies for use in treating disease.
Owner:ABZENA (CAMBRIDGE) LTD
Immunoconjugates with improved efficacy for the treatment of diseases
InactiveUS20070196274A1Improve therapeutic efficacyHigh detection sensitivityIn-vivo radioactive preparationsImmunoglobulins against animals/humansAntibody conjugateCD11a
The invention provides therapeutic or diagnostic antibodies with modified N— or C-terminal sequences that are enriched with lysine or tyrosine residues. These lysine or tyfosine residues can be used to couple radioisotopes, cytotoxic agents, or detectable labels. The increased stoichiometric ratios of these agents in the antibody conjugates lead to improved therapeutic efficacy or enhanced detection sensitivity. Non-limiting examples of antibodies suitable for the present invention include anti-CD22, anti-ErbB2, anti-VEGF, anti-EGFR, anti-VEGFR, anti-Her-3, anti-Her-4, anti-CEA, anti-CTLA-4, anti-CD4, anti-CD3, anti-CD20, anti-TNF-a, anti-CD11a, anti-Lewis Y antigen, anti-TrailR, anti-IL2R, anti-CD30, anti-CD146, anti-CD147, anti-alpha V integrin beta, anti-CD19, anti-GD2, anti-3H11, anti-EBV, anti-HIV, anti-HBV, anti-HCV, and other disease-specific antibodies.
Owner:WELSON PHARMA
Binding molecules specific for cd73 and uses thereof
ActiveUS20160194407A1Reduce proliferationEnhance immune responseAnimal cellsSugar derivativesDiseaseDrug conjugation
The present disclosure provides anti-CD73 binding molecules, e.g., antibodies and antigen binding fragments thereof. Also provided are pharmaceutical formulations comprising the disclosed compositions, and methods for the diagnosis and treatment of diseases associated with CD73-expression, e.g., cancer. Such diseases can be treated, e.g., by direct therapy with the anti-CD73 binding molecules disclosed herein (e.g., naked antibodies or antibody-drug conjugates that bind CD73), by adjuvant therapy with other antigen-binding anticancer agents such as immune checkpoint inhibitors (e.g., anti-CTLA-4 and anti-PD-1 monoclonal antibodies), and / or by combination therapies where the anti-CD73 molecules are administered before, after, or concurrently with chemotherapy.
Owner:MEDIMMUNE LTD
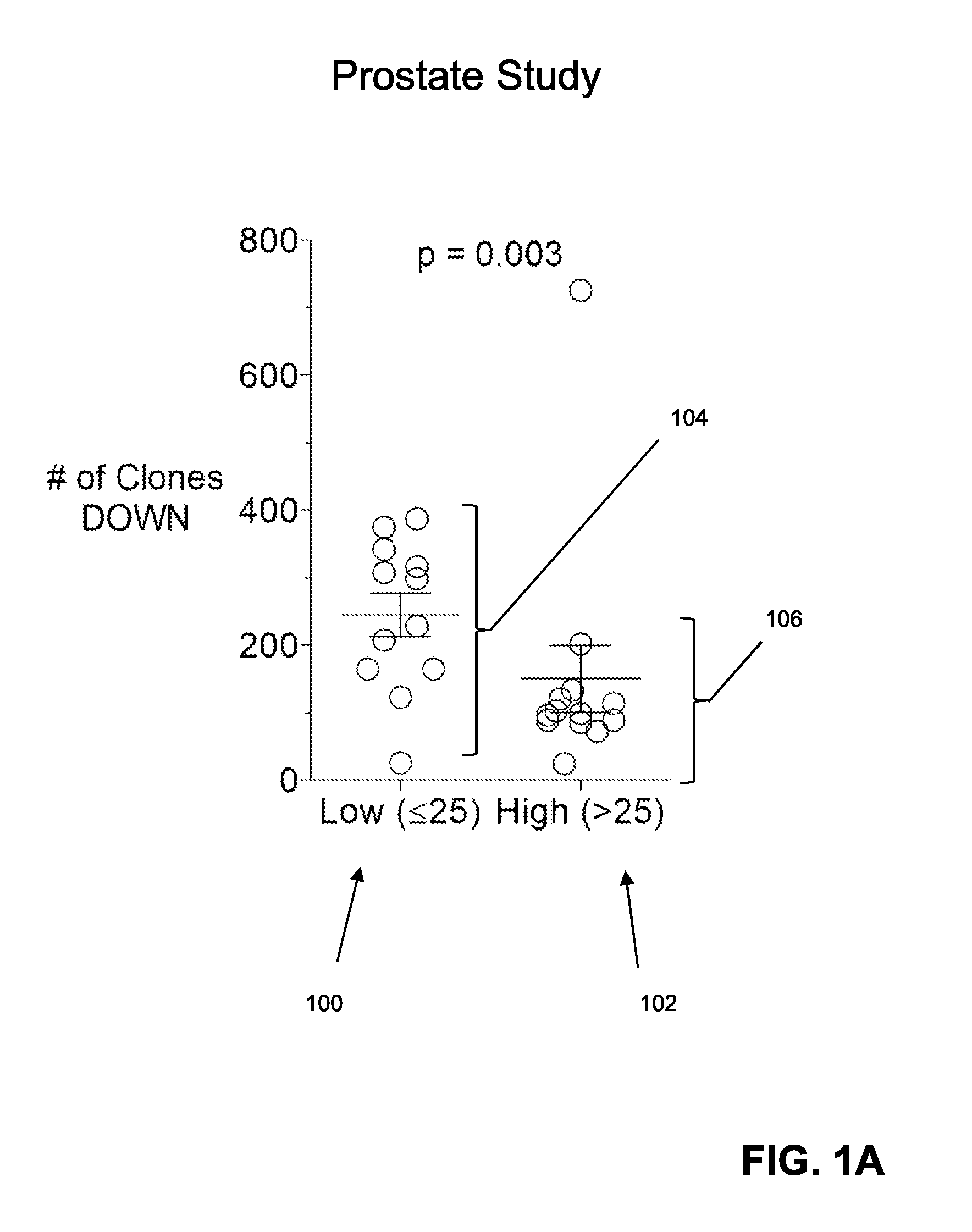
Predicting patient responsiveness to immune checkpoint inhibitors
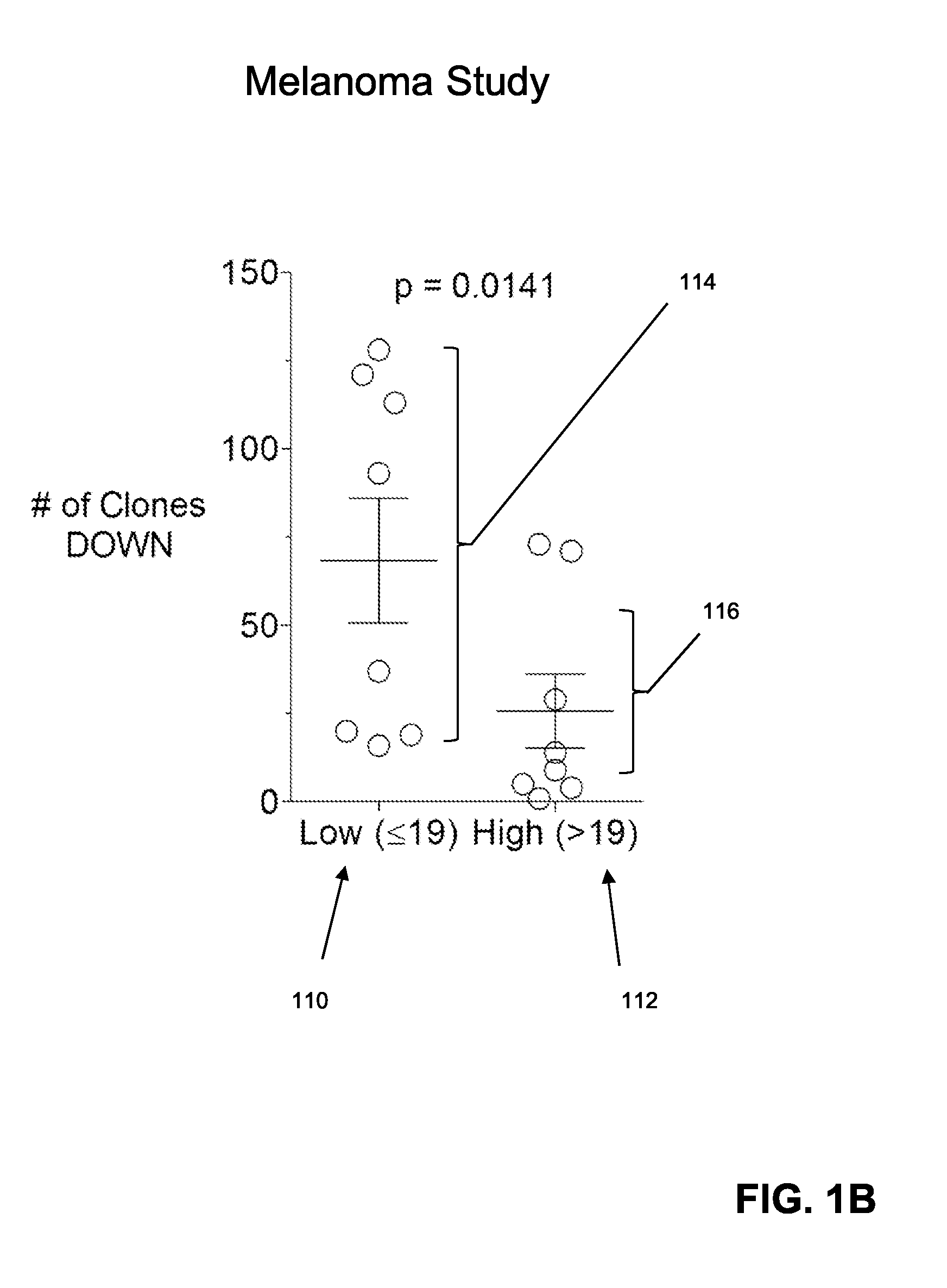
The invention is directed to a method of predicting clinical response of a patient to treatment of a cancer by an immune checkpoint pathway inhibitor, such as an anti-CTLA-4 or anti-PD-1 antibody binding compound. In one aspect the method comprises generating pre- and post-treatment clonotype profiles, determining a number of clonotypes that decrease in frequency between the first and second clonotype profiles, and predicting a lack of responsiveness in the patient to the treatment whenever the number of clonotypes that decrease in frequency is greater than a predetermined value.
Owner:ADAPTIVE BIOTECH +1
Monoclonal antibody and application thereof
ActiveCN101628940AMicroorganismsImmunoglobulins against cell receptors/antigens/surface-determinantsHeavy chainCTLA4 Protein
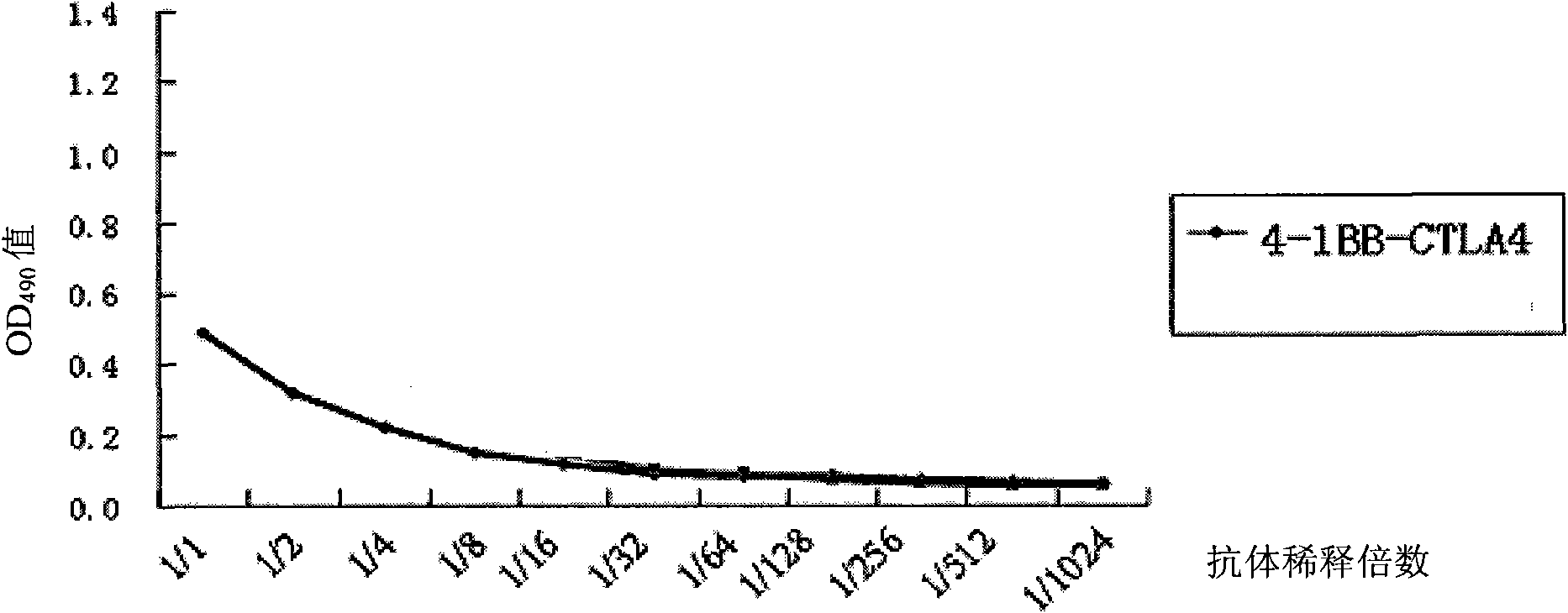
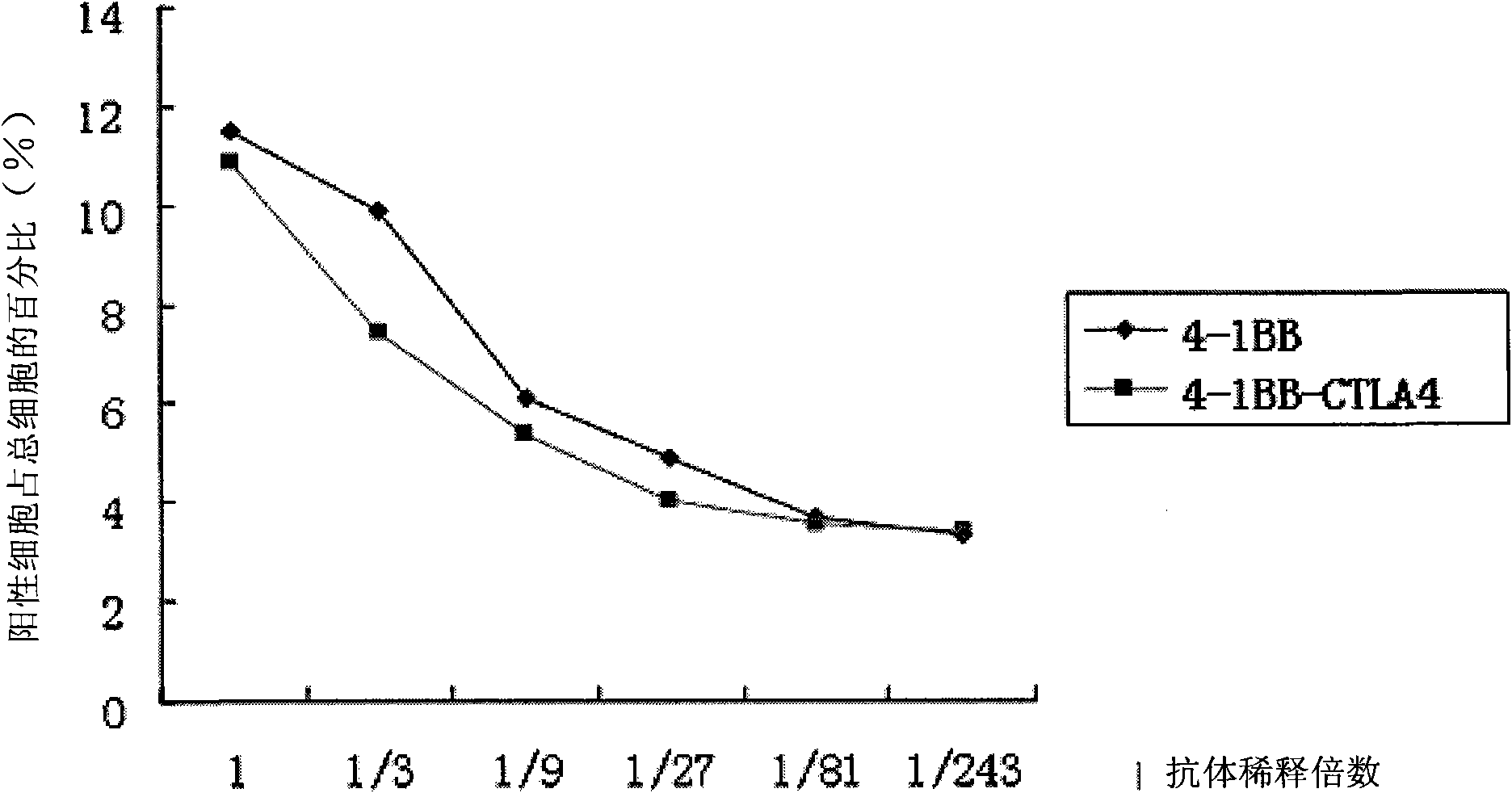
The invention discloses a monoclonal antibody and application thereof. The monoclonal antibody consists of light chains and heavy chains, wherein amino acid residue sequences of the variable area of the heavy chains are showed in a sequence 1 in the sequence table, and amino acid residue sequences of the variable area of the light chains are showed in a sequence 2 in the sequence table. The invention combines and constructs anti-CTLA-4 antibodies and anti-4-1BB antibodies to form antihuman 4-1BB and CTLA-4 monoclonal antibodies. Experimental result shows that the monoclonal antibody has an antibody structure and can be respectively combined with human 4-1BB and CTLA-4.
Owner:广东云天抗体生物科技有限公司
Oncolytic adenoviral vectors coding for monoclonal Anti-ctla-4 antibodies
InactiveUS20130243731A1Increased E2F levelImprove the level ofBiocideGenetic material ingredientsOncolytic adenovirusApoptosis
The present invention relates to the fields of life sciences and medicine. Specifically, the invention relates to cancer therapies. More specifically, the present invention relates to oncolytic adenoviral vectors and cells and pharmaceutical compositions comprising said vectors. The present invention also relates to said vectors for treating cancer in a subject and a method of treating cancer in a subject. Furthermore, the present invention relates to methods of producing monoclonal anti-CTLA4 antibodies in a cell and increasing tumor specific immune response and apoptosis in a subject, as well as uses of the oncolytic adenoviral vectors for producing monoclonal anti-CTLA4 antibodies in a cell and increasing tumor specific immune response and apoptosis in a subject.
Owner:ONCOS THERAPEUTICS
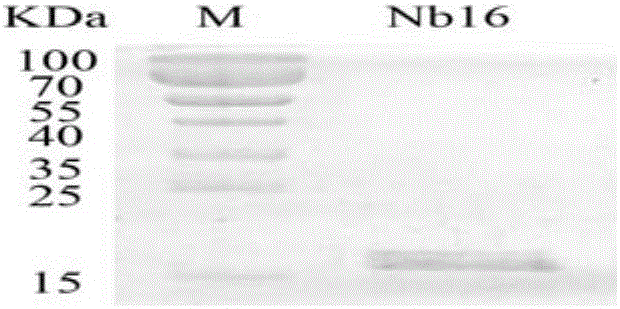
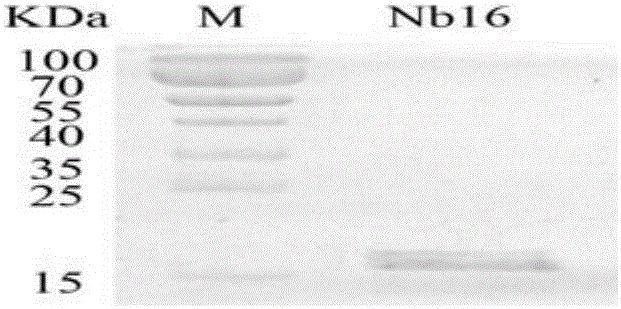
Nanometer antibody Nb16 for anti-CTLA-4 and preparation method and application of nanometer antibody Nb16
ActiveCN106220732AEfficient expressionPromote proliferationMaterial nanotechnologyImmunoglobulins against cell receptors/antigens/surface-determinantsCTLA4 ProteinBiology
The invention discloses a nanometer antibody Nb16 for anti-CTLA-4 and a preparation method and application of the nanometer antibody Nb16. The provided nanometer antibody comprises a determinant complementary region and a frame region. The determinant complementary region of the nanometer antibody includes CDR1, CDR2 and CDR3, wherein the amino acid sequence of CDR1 is the 26-35 amino acid of SEQ ID No.1 in a sequence list, the amino acid sequence of CDR2 is 51<st>-59 amino acid of SEQ ID No.1 in a sequence list, and the amino acid sequence of CDR3 is 97-106 amino acid of SEQ ID No.1 in a sequence list. The nanometer antibody Nb16 can combine with T cells and CTLA-4, and can be used for researching and developing a CTLA-4 molecular detection agent, preparing a tumor inhibitor or a tumor cell inhibitor, and preparing a medicine for inhibiting activity of CTLA-4 and prompting T cell proliferation.
Owner:GUANGXI MEDICAL UNIVERSITY
Humanised anti CTLA-4 antibodies
The invention provides an anti-CTLA4 antibody which inhibits the binding of CTLA4 to human B7, in particular, it inhibits binding of CTLA4 to human B7.1 and / or human B7.2. Specific antibodies are provided with specific variable region sequences as well as compositions comprising such antibodies for use in treating disease.
Owner:ABZENA (CAMBRIDGE) LTD
TNF-alpha blocker treatment for enterocolitis associated with immunostimulatory therapeutic antibody therapy
Owner:ER SQUIBB & SONS INC +1
Anti-b7-h1 and Anti-ctla-4 antibodies for treating non-small cell lung cancer
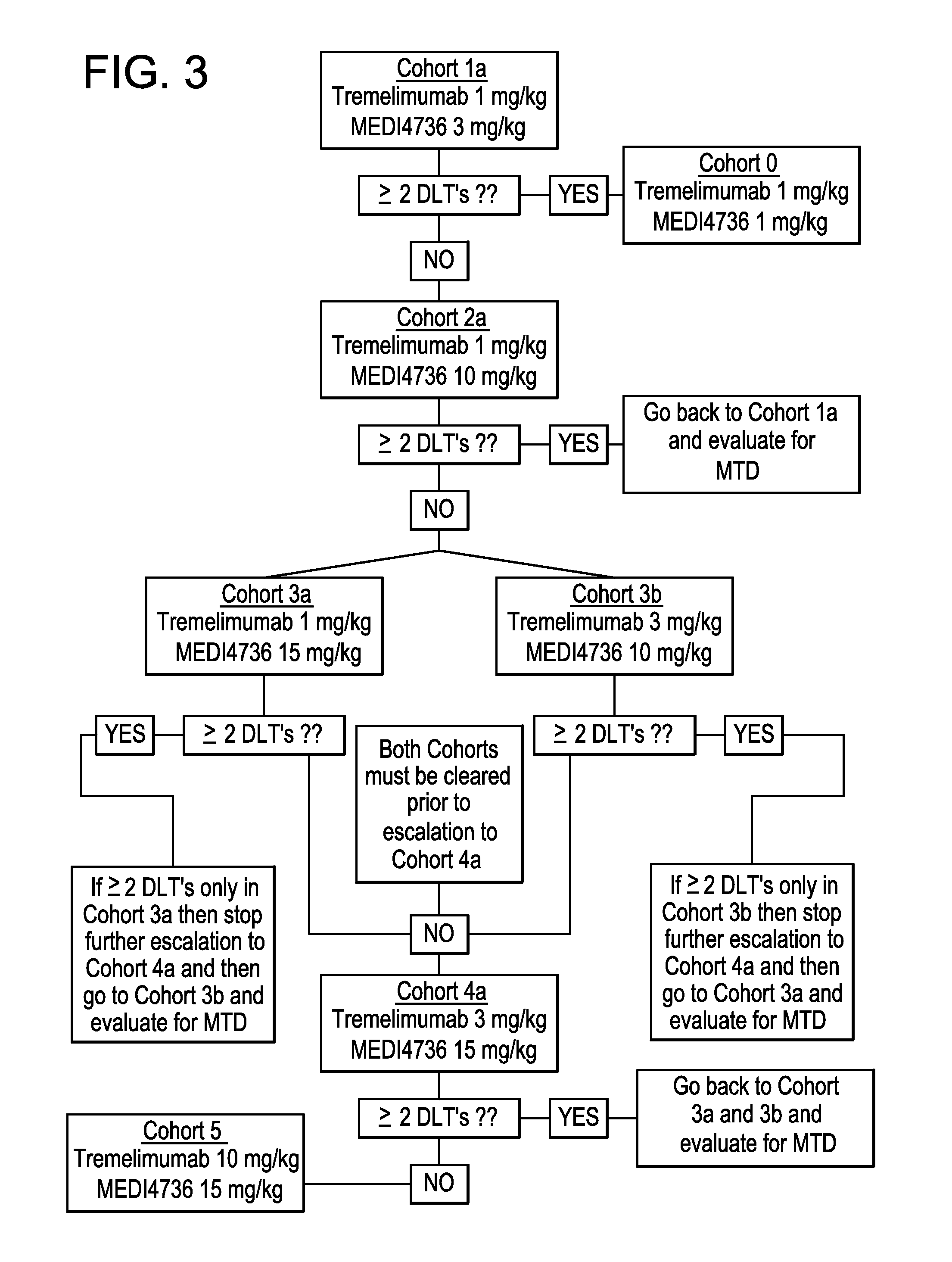
ActiveUS20150328311A1Prolonged progression-free survivalImprove survivalAntibody ingredientsImmunoglobulinsAntigenAntigen Binding Fragment
Provided herein are methods of treating non-small cell lung cancers comprising administering an effective amount of MEDI4736 or an antigen-binding fragment thereof and tremelimumab or an antigen-binding fragment thereof.
Owner:MEDIMMUNE LLC
Anti-ctla-4 and cpg-motif-containing synthetic oligodeoxynucleotide combination therapy for cancer treatment
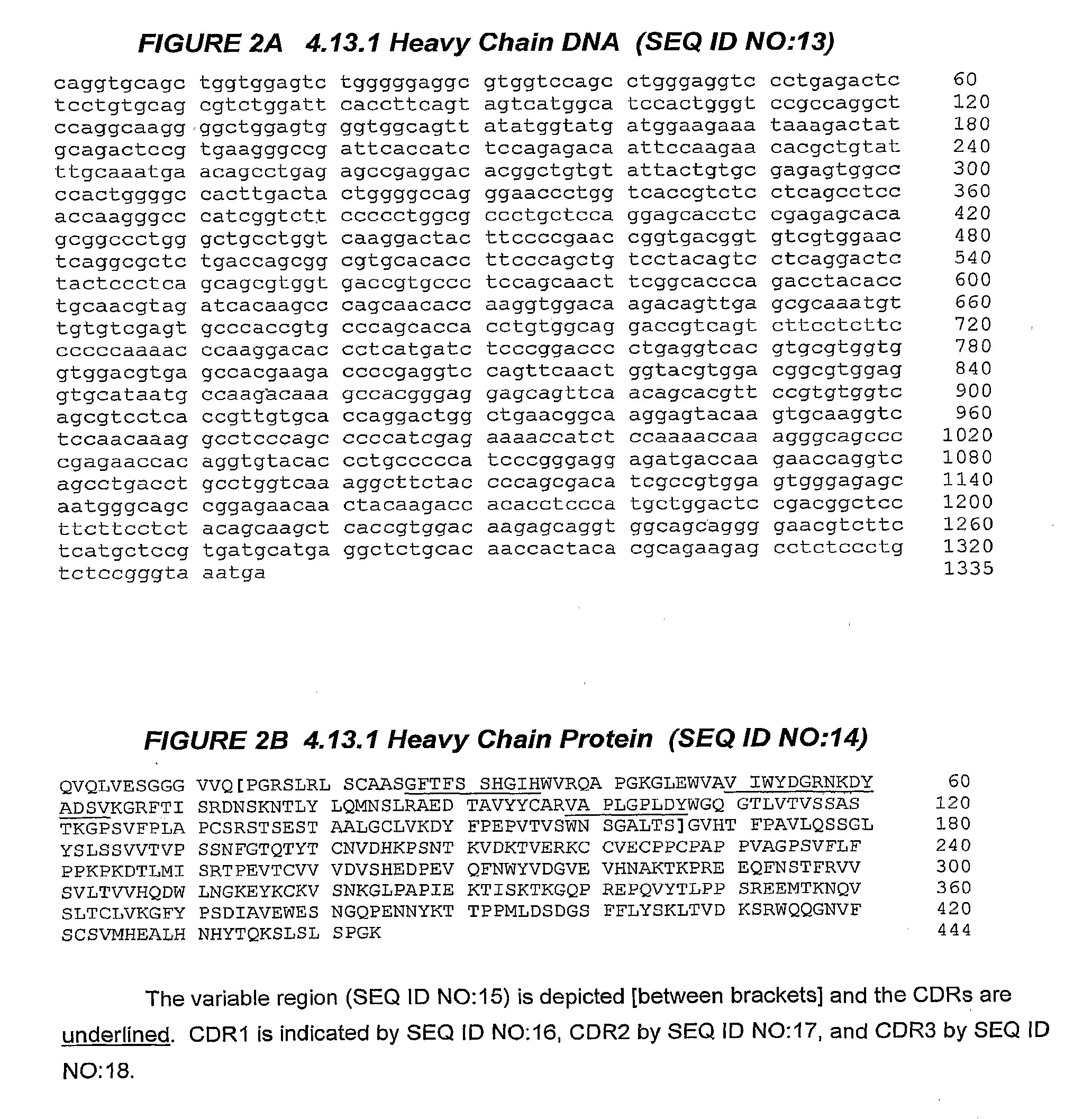
The invention relates to administration of an anti-CTLA-4 antibody, particularly human antibodies to human CTLA-4, such as those having amino acid sequences of antibodies 3.1.1, 4.1.1, 4.8.1, 4.10.2, 4.13.1, 4.14.3, 6.1.1, 11.2.1, 11.6.1, 11.7.1, 12.3.1.1, 12.9.1.1, and MDX-010, in combination with an immunostimulatory nucleotide, i.e., CpG ODN PF3512676, for treatment of cancer. The invention relates to administering a combination of an anti-CTLA-4 antibody and CpG ODN PF3512676 as neoadjuvant, adjuvant, first-line, second-line, and third-line therapy of cancer, whether localized or metastasized, and at any point(s) along the disease continuum (e.g., at any stage of the cancer).
Owner:COLEY PHARMA GRP INC +1
Binding molecules specific for CD73 and uses thereof
ActiveUS9938356B2Reduce proliferationEnhance immune responseImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsDiseaseAnticarcinogen
The present disclosure provides anti-CD73 binding molecules, e.g., antibodies and antigen binding fragments thereof. Also provided are pharmaceutical formulations comprising the disclosed compositions, and methods for the diagnosis and treatment of diseases associated with CD73-expression, e.g., cancer. Such diseases can be treated, e.g., by direct therapy with the anti-CD73 binding molecules disclosed herein (e.g., naked antibodies or antibody-drug conjugates that bind CD73), by adjuvant therapy with other antigen-binding anticancer agents such as immune checkpoint inhibitors (e.g., anti-CTLA-4 and anti-PD-1 monoclonal antibodies), and / or by combination therapies where the anti-CD73 molecules are administered before, after, or concurrently with chemotherapy.
Owner:MEDIMMUNE LTD
Anti-CTLA-4 nano antibody Nb91 and preparation method and application thereof
ActiveCN106188297AEfficient expressionPromote proliferationMaterial nanotechnologyImmunoglobulins against cell receptors/antigens/surface-determinantsWilms' tumorTumor cells
The invention discloses an anti-CTLA-4 nano antibody Nb91 and a preparation method and an application thereof. The nano antibody provided by the invention includes a determinant complementary region and a frame region; the determinant complementary region of the nano antibody consists of CDR1, CDR2 and CDR3; an amino acid sequence of the CDR1 comprises 26th-32nd amino acids of SEQ ID NO.7 in a sequence table; an amino acid sequence of the CDR2 comprises 48th-55th amino acids of SEQ ID NO.7 in the sequence table; an amino acid sequence of the CDR3 comprises 94th-106th amino acids of SEQ ID NO.7 in the sequence table. The nano antibody Nb91 can be combined with T cells and CTLA-4, and can be applied to research and development of CTLA-4 molecular detection reagents, preparation of tumor inhibitors or tumor cell inhibitors and preparation of drugs for inhibition of CTLA-4 activity and promotion of T cell proliferation.
Owner:GUANGXI MEDICAL UNIVERSITY
Tnf-alpha blocker treatment for enterocolitis associated with immunostimulatory therapeutic antibody therapy
The present invention provides methods for treating adverse events related to immunotherapy. More specifically, the present invention provides methods for treating the enterocolitis associated with anti-CTLA-4 antibody immunotherapy.
Owner:MEDAREX LLC +1
Compositions comprising coformulation of Anti-pd-l1 and Anti-ctla-4 antibodies
ActiveUS20170306025A1Immunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsAntigen Binding FragmentPD-L1
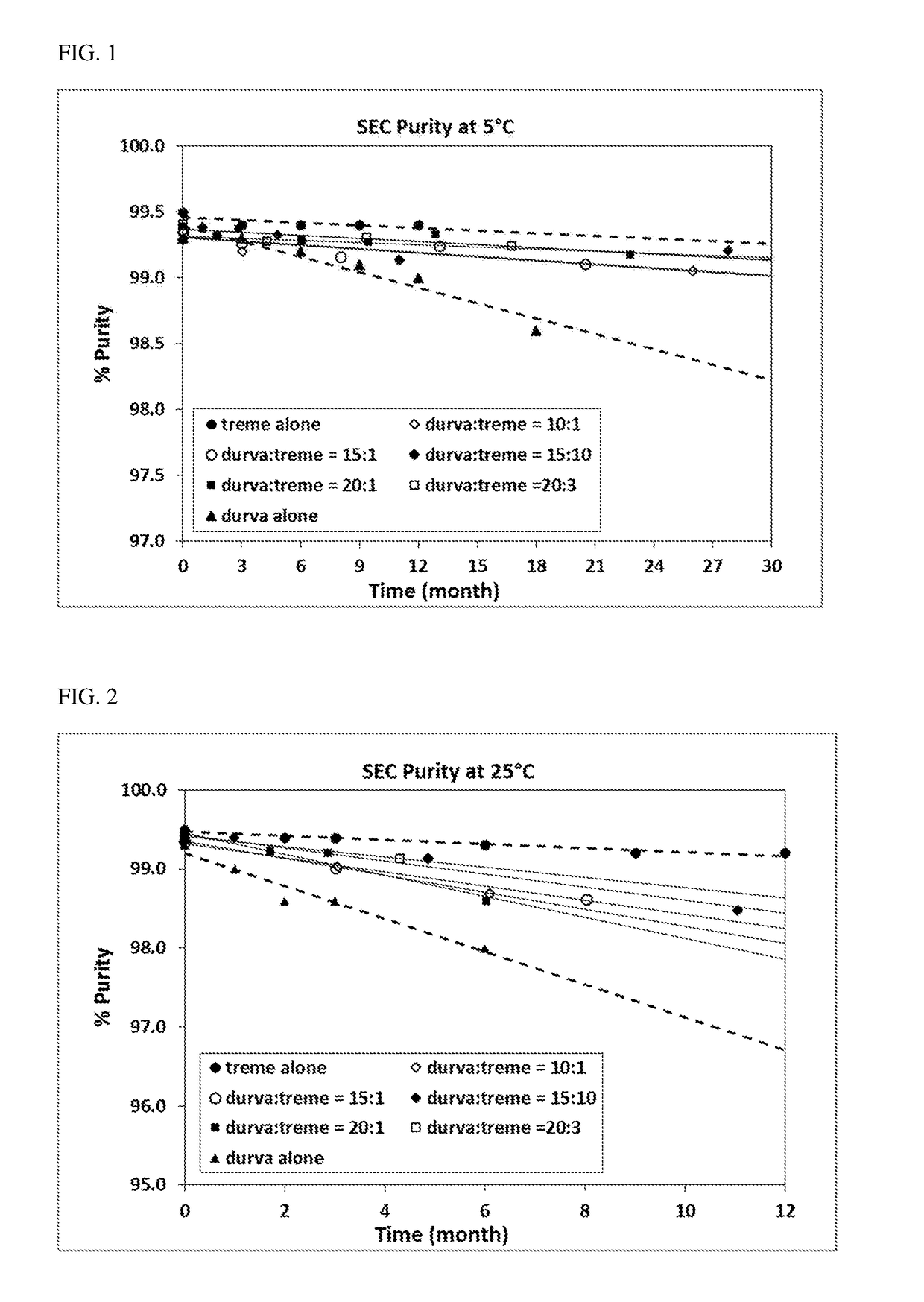
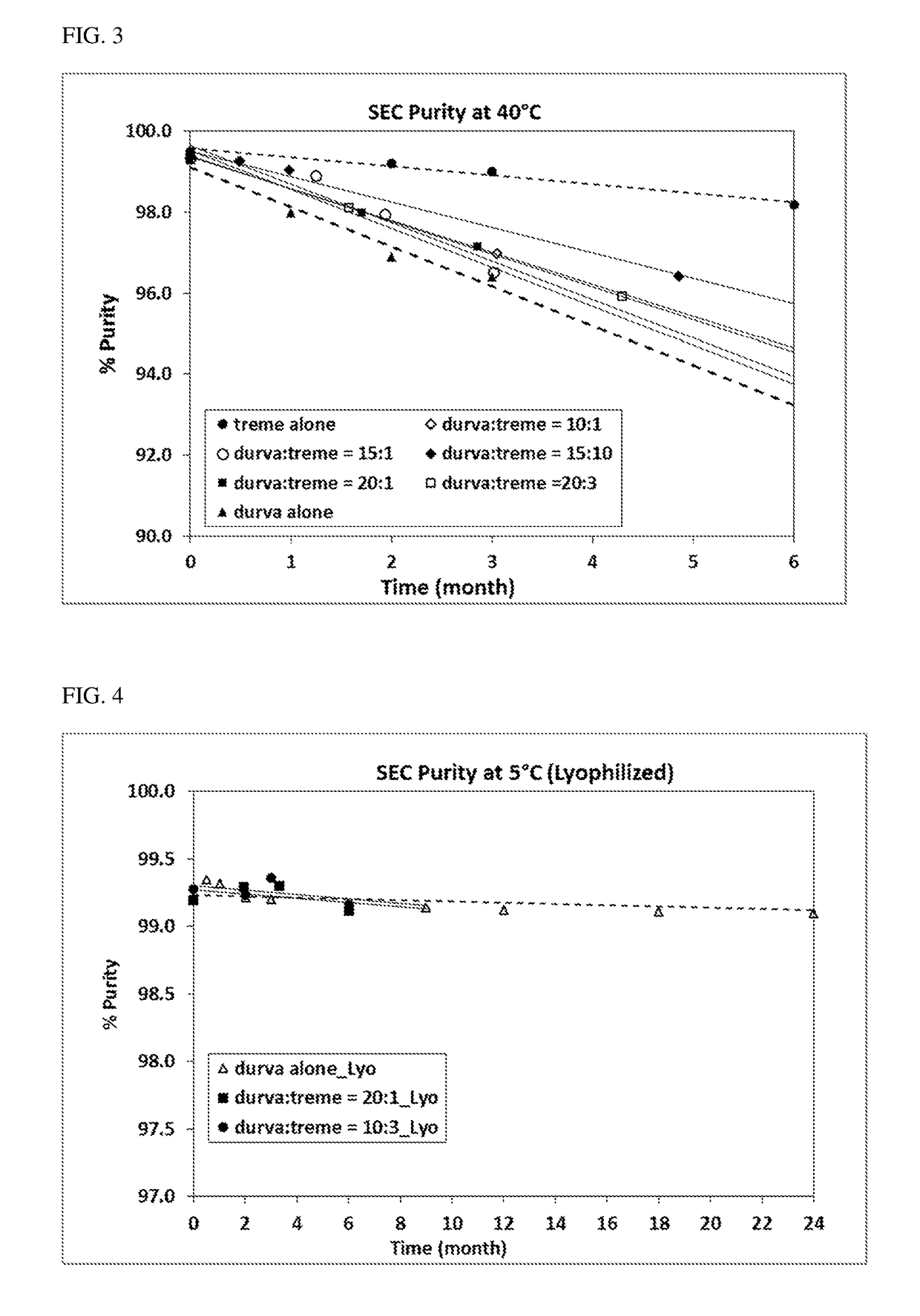
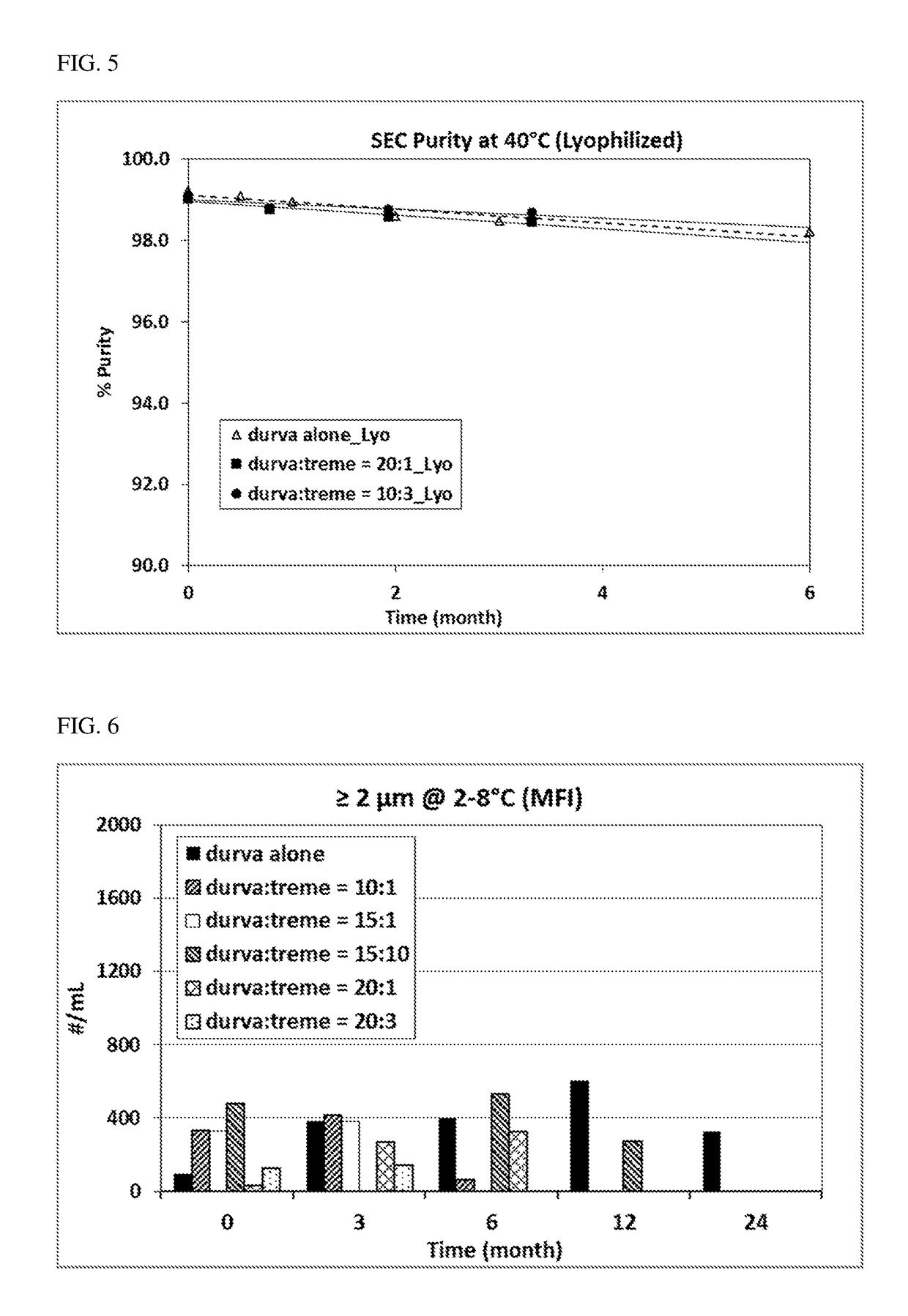
Provided herein are compositions comprising coformulation of anti-PD-L1 and anti-CTLA-4 antibodies, or antigen-binding fragments thereof, and methods of making and using such compositions. In various aspects, stable coformulations of the anti-PD-L1 antibody durvalumab (MEDI4736) and the anti-CTLA-4 antibody tremelimumab are provided.
Owner:MEDIMMUNE LLC
Treatment of PD-L1-Negative Melanoma Using an Anti-PD-1 Antibody and an Anti-CTLA-4 Antibody
ActiveUS20160340428A1Shrink tumorIncreasing objective response rateBiological material analysisImmunoglobulins against cell receptors/antigens/surface-determinantsProgression-free survivalMelanoma
The invention provides a method of treating a melanoma comprising (i) identifying a patient having a PD-L1-negative melanoma and (ii) administering to the patient a combination of an anti-PD-1 antibody or an antigen-binding portion thereof and an anti-CTLA-4 antibody or an antigen-binding portion thereof. The methods of the invention can extend progression-free survival for over 8 months and / or reduces the tumor size at least about 10%, about 20%, about 30%, about 40%, or about 50% compared to the tumor size prior to the administration.
Owner:BRISTOL MYERS SQUIBB CO
Anti-ctla-4 antibodies
ActiveUS20170226211A1Recovery functionPolypeptide with localisation/targeting motifAntibody mimetics/scaffoldsAntibodyAnti ctla 4
Owner:AGENCY FOR SCI TECH & RES
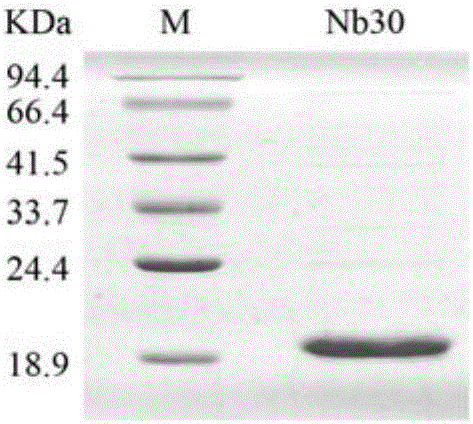
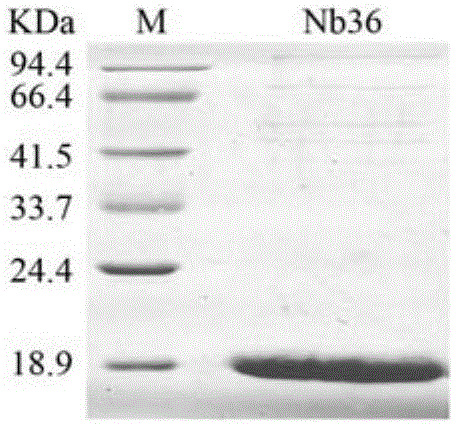
Anti-CTLA-4 nanobody Nb30 as well as preparation method and application thereof
ActiveCN106046165AEfficient expressionPromote proliferationMaterial nanotechnologyImmunoglobulins against cell receptors/antigens/surface-determinantsComplementarity determining regionWilms' tumor
The invention discloses an anti-CTLA-4 nanobody Nb30 as well as a preparation method and application thereof. The nanobody provided by the invention comprises a complementarity-determining region and a framework region; the complementarity-determining region of the nanobody consists of CDR1, CDR2 and CDR3; the amino acid sequence of CDR1 is that of a 26th-35th amino acid of SEQ ID No. 3 in a sequence table; the amino acid sequence of CDR2 is that of a 51st-58th amino acid of SEQ ID No. 3 in the sequence table; the amino acid sequence of CDR3 is that of a 97th-115th amino acid of SEQ ID No. 3 in the sequence table. The nanobody Nb30 can be combined to T cells and CTLA-4, can be used for research and development of a CTLA-4 molecular detection reagent, and can be used for preparing a tumor inhibitor or a tumor cell inhibitor and preparing drugs for inhibiting CTLA-4 activity and promoting proliferation of the T cells.
Owner:GUANGXI MEDICAL UNIVERSITY
Activatable Anti-CTLA-4 Antibodies and Uses Thereof
ActiveUS20190359714A1Reduce the binding forceEliminate side effectsAntibody mimetics/scaffoldsBiological material analysisHeavy chainTumor microenvironment
Provided herein are activatable anti-human CTLA-4 antibodies comprising a heavy chain comprising a VH domain and a light chain comprising a masking moiety (MM), a cleavable moiety (CM), and a VL domain. Such activatable anti-human CTLA-4 antibodies have CTLA-4 binding activity in the tumor microenvironment, where the masking moiety is removed by proteolytic cleavage of the cleavable moiety by tumor-specific proteases, but exhibit greatly reduced binding to CTLA-4 outside the tumor. In this way, the activatable anti-human CTLA-4 antibodies of the present invention retain anti-tumor activity while reducing the side effects associated with anti-CTLA-4 activity outside the tumor.
Owner:CYTOMX THERAPEUTICS +1
Ipilimumab variant with enhanced ADCC
ActiveUS10196445B1Enhanced ADCC activityImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsHeavy chainIpilimumab
The present invention provides anti-CTLA-4 antibodies having enhanced ADCC activity, and their use in treating cancer. In one embodiment, the anti-CTLA-4 antibody is ipilimumab, and ADCC activity is enhanced by introducing G236A, S239D, A330L and I332E mutations (“GASDALIE”) into the Fc region of the heavy chain constant domain.
Owner:BRISTOL MYERS SQUIBB CO
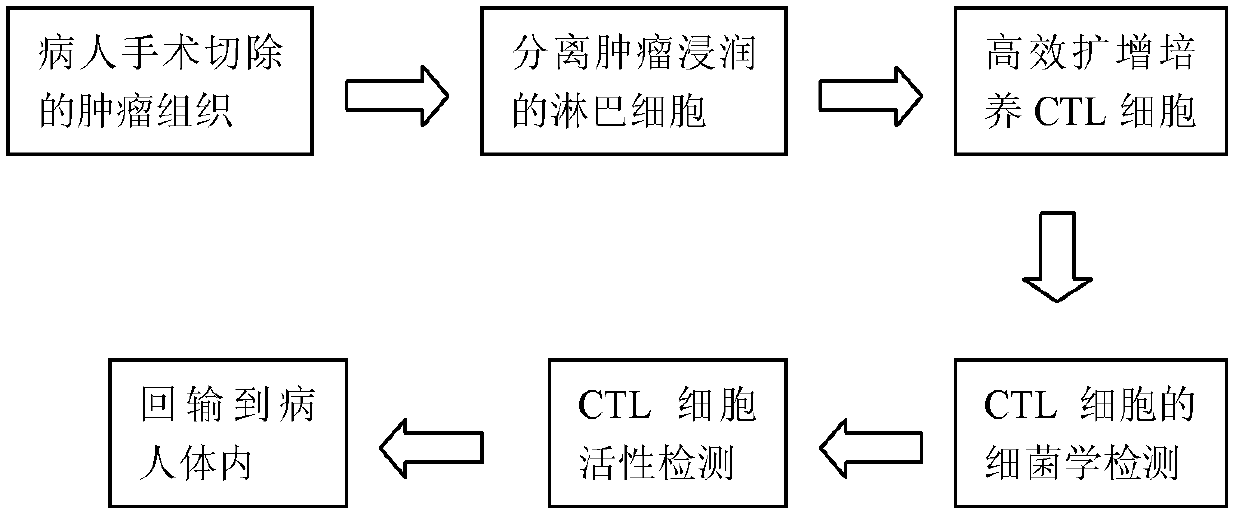
High-efficiency amplification culture solution for tumor infiltration lymphocytes
InactiveCN103374548AImprove efficiencyInhibitory inhibitory functionBlood/immune system cellsLymphocyteT lymphocyte
The invention provides a high-efficiency amplification culture solution for tumor infiltration lymphocytes. The culture solution contains IL-2, IL-12, anti-CD28 and anti-CTLA-4. Compared with the prior art, the high-efficiency amplification culture solution has the advantages that a combined serum-free culture medium containing IL-2, IL-12, anti-CD28, anti-CTLA-4 and other cell factors is adopted, the amplification efficiency of TIL cells is obviously improved, and the amplification efficiency is about 3-10 times higher than the amplification efficiency of a common amplification culture solution; moreover, an inhibitory function of Treg lymphocytes on cytotoxic T lymphocytes can be effectively achieved, and the amplified lymphocyte has an extremely strong tumor killing effect.
Owner:SHANGHAI NUOHAOYA MEDICAL DIAGNOSTICS TECH CO LTD
Methods and Compositions for Localized Secretion of Anti-CTLA-4 Antibodies
ActiveUS20170114364A9Reduce functionReduce the binding forceOrganic active ingredientsBiocideSecretionTarget tissue
The present invention provides compositions and methods for effectuating the localized expression of anti-CTLA-4 antibody proximal to a target tissue in a patient.
Owner:RGT UNIV OF CALIFORNIA
Low dose immune checkpoint blockade in metastatic cancer
InactiveUS20170253655A1Safely exploitedGood effectOrganic active ingredientsPeptide/protein ingredientsSide effectIl-2 therapy
A method of treating cancer comprising and reducing autoimmune side effects in administration of anti-CTLA-4 antibodies. The invention provides methods for low dose immune checkpoint (IC) treatment of metastatic cancer by delivering anti-CTLA-4 and anti-PD-1 antibodies to cancer patients. Methods also provide for IL-2 stimulation for the activation of T cells against tumor cells. The invention further provides methods for daily cyclic high fever response (hyperthermia) during IL-2 therapy. The methods provide treatment of metastatic cancer without unacceptable autoimmune side effects.
Owner:KLEEF RALF
Use of Anti-ctla-4 antibodies with enhanced adcc to enhance immune response to a vaccine
InactiveUS20190382490A1Enhance ADCCEnhances binding to activating Fcγ receptorsViral antigen ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsFucosylationIpilimumab
Owner:BRISTOL MYERS SQUIBB CO
Methods of treating cancer using il-21 and monoclonal antibody therapy
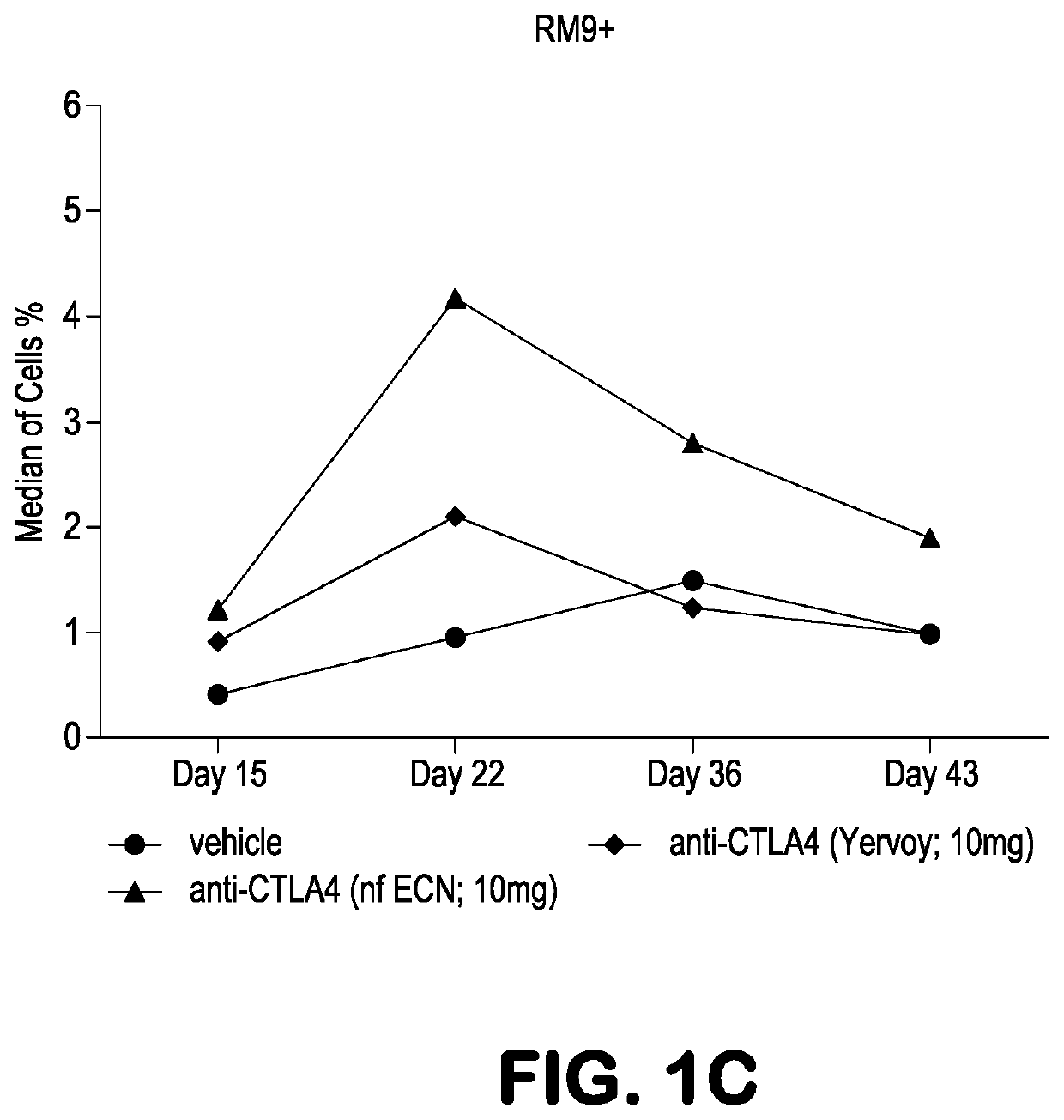
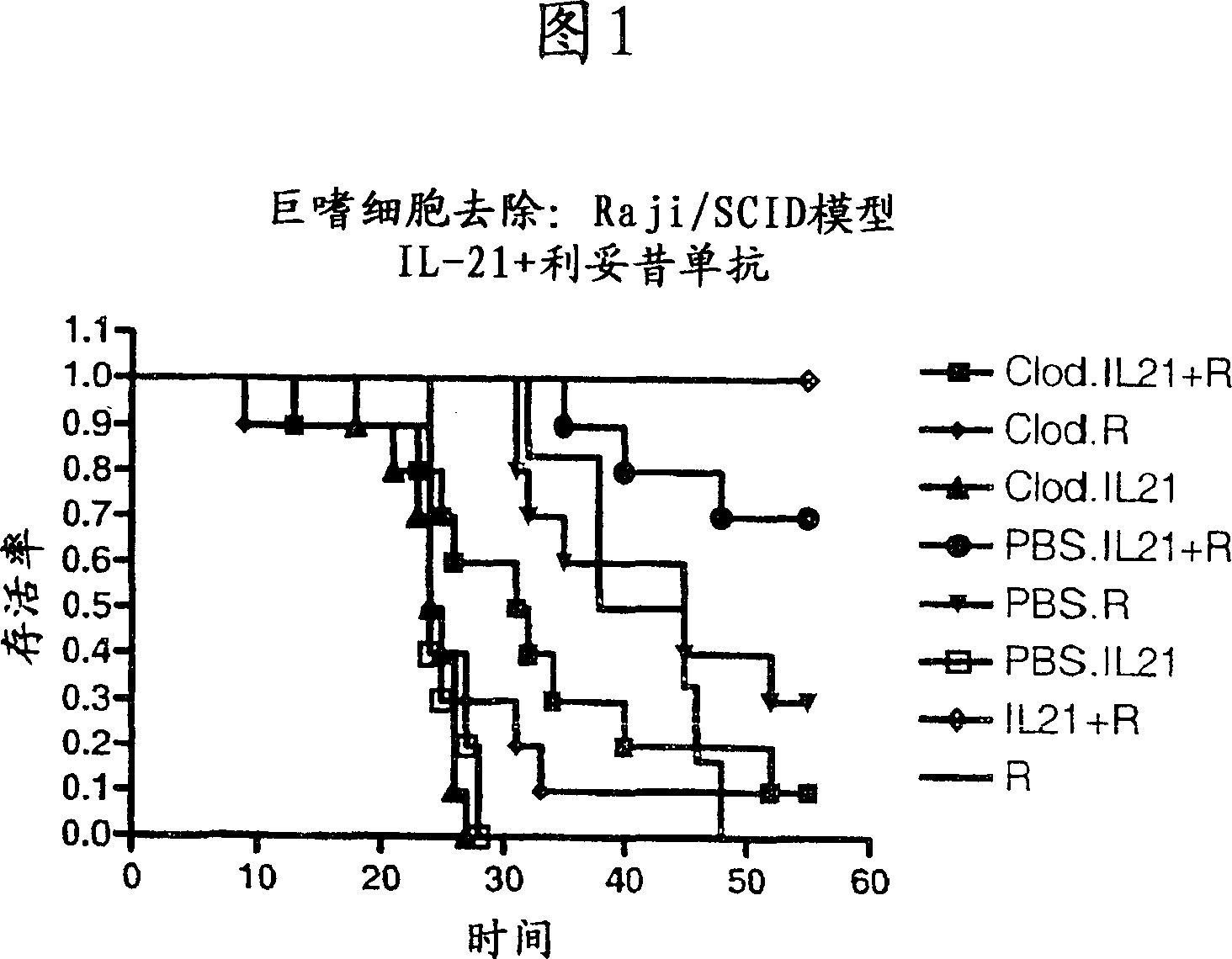
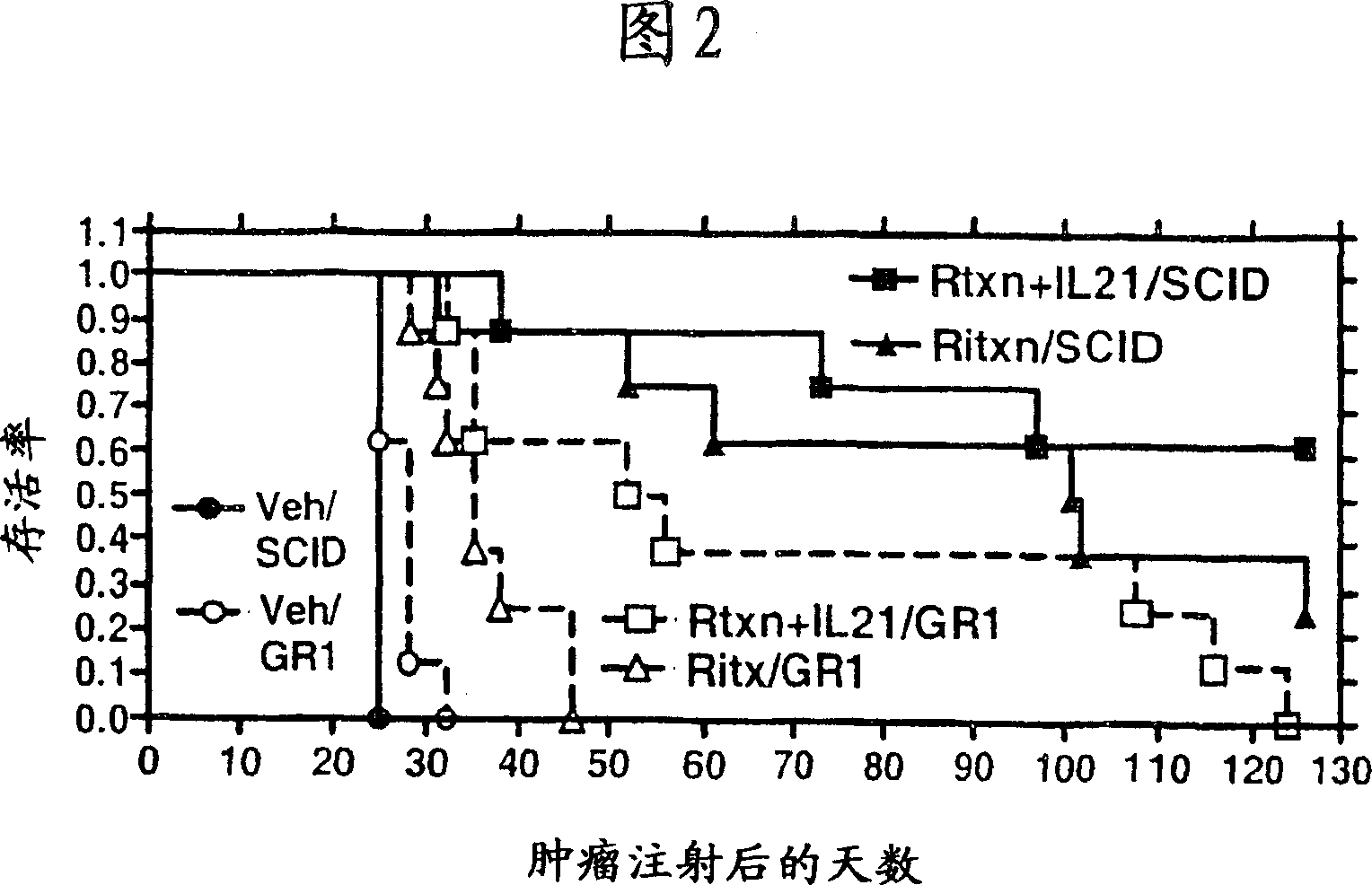
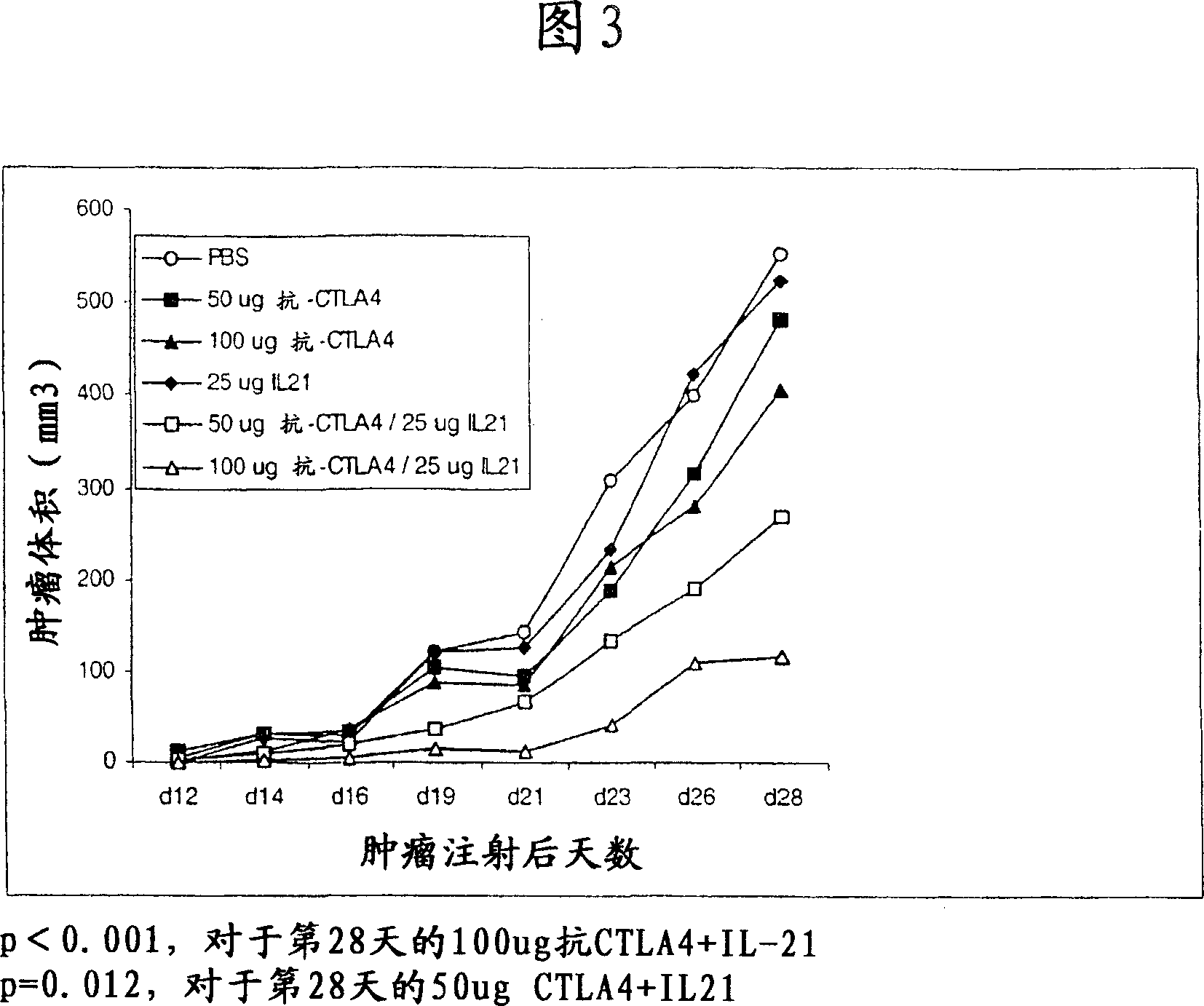
A method of treating cancer by co-administering a therapeutic monoclonal antibody and IL-21 is described. Exemplary monoclonal antibodies that may be used are rituximab, trastuzumab, and anti-CTLA-4 antibodies. For patient populations resistant to monoclonal antibody therapy, relapsed after treatment with monoclonal antibodies, or in which enhanced IL-21 antitumor effects reduce toxicity associated with therapy with monoclonal antibodies, the The enhanced antitumor properties of the combination therapy described above are particularly useful.
Owner:ZYMOGENETICS INC
Therapeutic combinations and methods for treating neoplasia
InactiveUS20170320954A1Organic active ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsAbnormal tissue growthPD-L1
The disclosure features a CXCR2 antagonist in combination with a checkpoint inhibitor (e.g., an anti-CTLA-4 antibody or an anti-PD-L1 antibody) and methods of using the combination to enhance anti-tumor activity in a subject.
Owner:MEDIMMUNE LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com