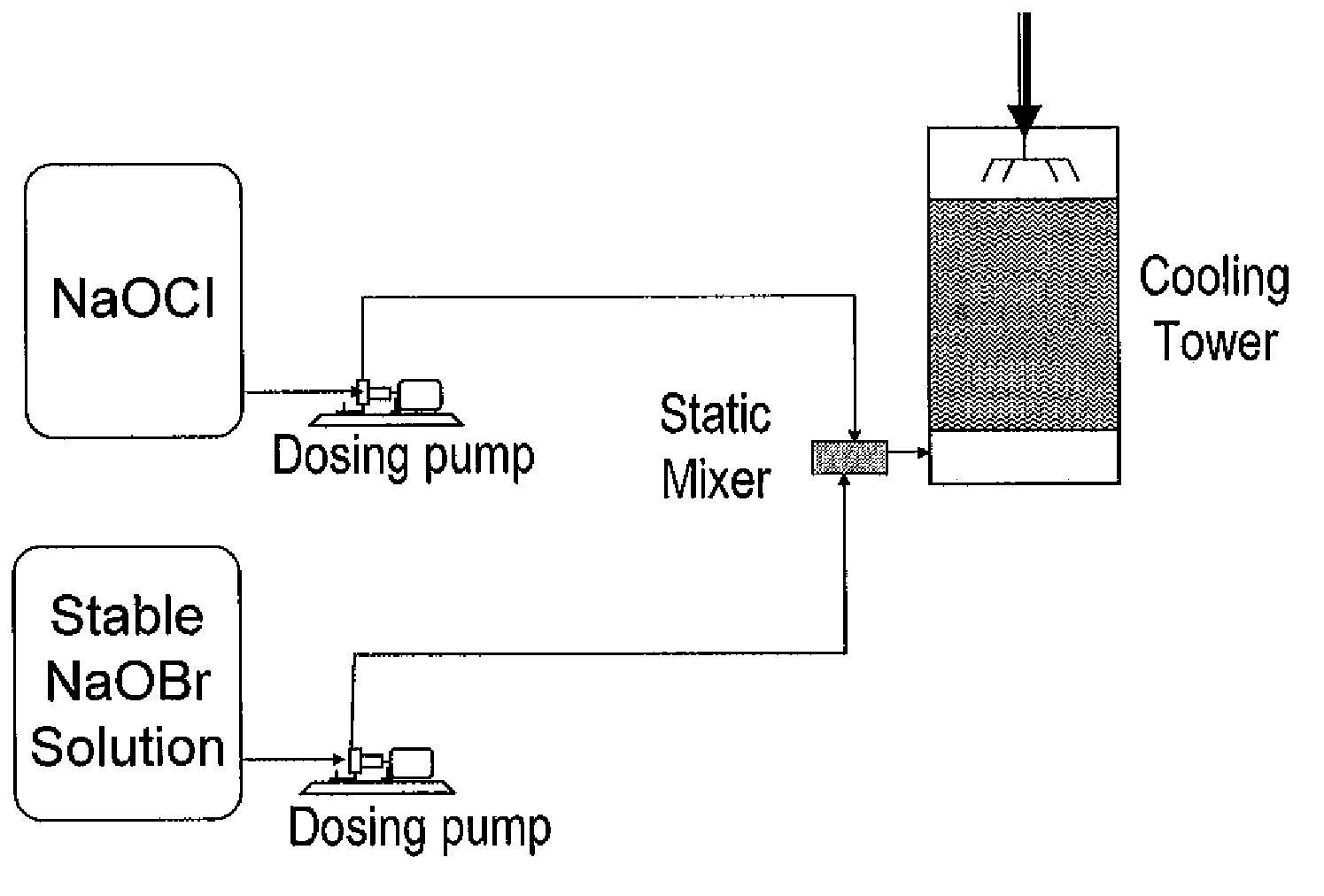
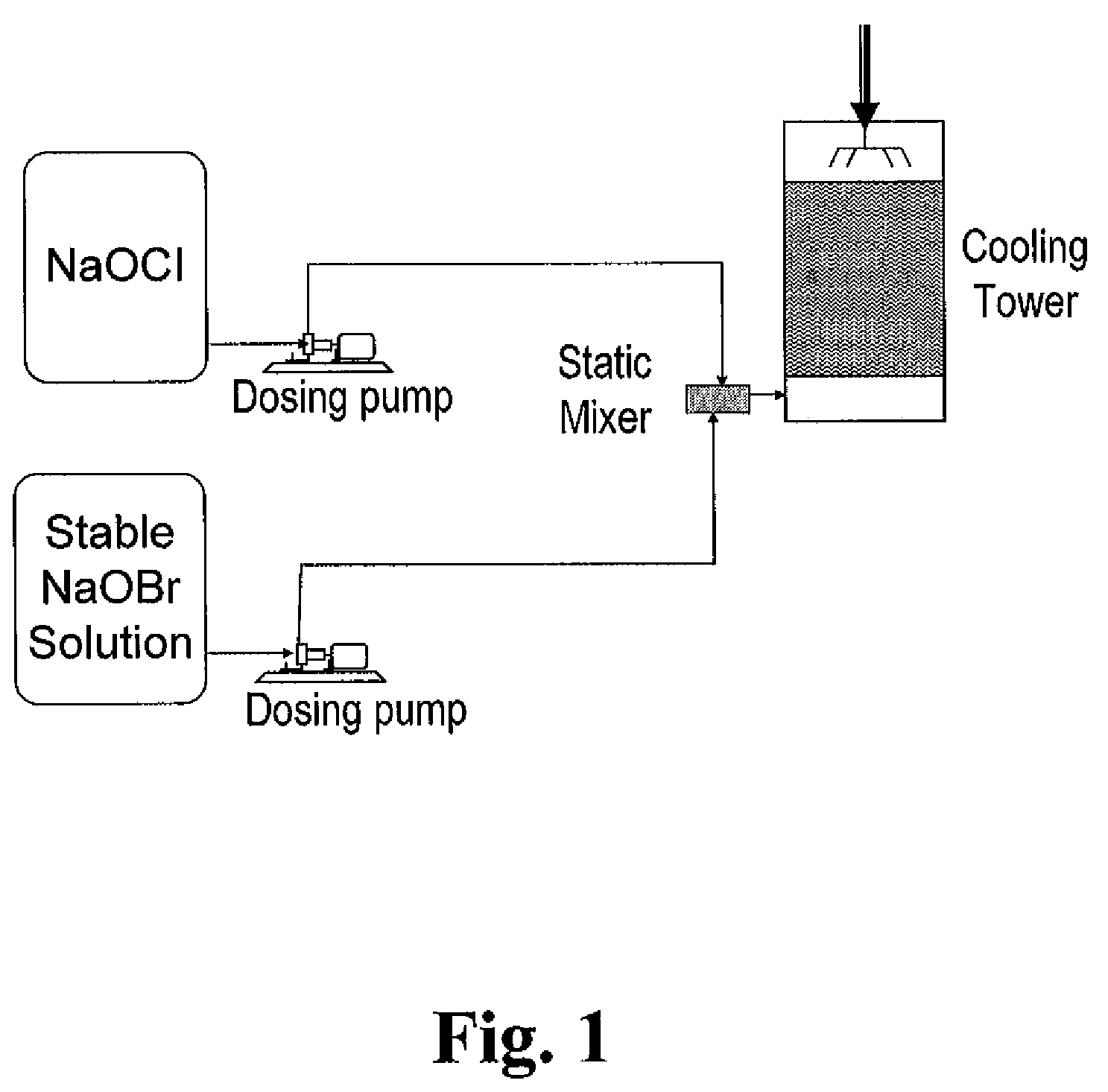
Process for the preparation of concentrated solutions of stabilized hypobromites
a concentrated solution and stabilized technology, applied in the field of concentrated solution preparation of stabilized hypobromites, can solve the problems of slow precipitation, insufficient stable for practical and commercial application, and the disadvantage of yielding equivalent amounts of naobr and nacl of hypochlorite, and achieve the effect of higher stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0035]A concentrated hypobromite solution was prepared by contacting, under vigorous stirring, a mixture of 532.5 g of a concentrated aqueous NaOH solution (49.2 wt %) and 494 g water with 480 g bromine, while adding gradually so that the temperature is maintained at 0±5° C. The NaOH / bromine molar ratio was 2.2:1. A clear, dark yellow solution of unstabilized sodium hypobromite was obtained, which contained 23.2 wt % NaOBr and 20.5 wt % NaBr. In spite of the very high concentration and low temperature, no precipitation occurred due to the very high solubility of NaBr. To the above hypobromite solution, an aqueous solution of sodium sulfamate was added gradually, in order to keep the temperature between −5 to 10° C., preferably 0-5° C.
[0036]The above said aqueous solution of sodium sulfamate was prepared by gradually adding at room temperature 401.7 g of an aqueous 49.2 wt % NaOH solution to 836.5 g of an aqueous slurry composed of 436.5 g sulfamic acid and 400 g water, was added to ...
example 2
[0038]In a reactor provided with cooling jacket and mixing device, introduced was 500 g stabilized bromine solution prepared as described in Example 1. To this solution, cooled at 0-5° C., was added 377 g of a sodium hypochlorite commercial solution containing 11% sodium hypochlorite (expressed as available chlorine) that reacted with the NaBr present, forming NaOBr. The solution in the reactor (877 g) then contained ca 14.6% NaOBr, practically no NaBr, 4.6% NaCl and 10.9% sulfamate. The solution can be utilized as such but it is less stable on storage because the ratio between sulfamate and hypobromite is less than required. This solution contained 8.8% available halogen (as Cl2).
example 3
[0039]A more stable, but less concentrated, stabilized bromine solution was prepared by adding, at 0-10° C., to the mixture prepared as in Example 2, 222 g sodium sulfamate, prepared as in Example 1. The 1109 g solution thus obtained contained ca 11.5 wt % sodium hypobromite. In terms of available chlorine it contained ca 7% available chlorine. The molar ratio of sodium sulfamate to NaOBr in this solution was 1.5:1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperatures | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com