Adrenomedullin production enhancer
a technology of adrenomedullin and enhancing agent, which is applied in the direction of biocide, cardiovascular disorder, drug composition, etc., can solve the problems of high production cost, inability to exert the effect of significant amount, and inability to administer significant amount, so as to increase the amount of blood flow, promote the production of adrenomedullin, and increase the effect of blood flow
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
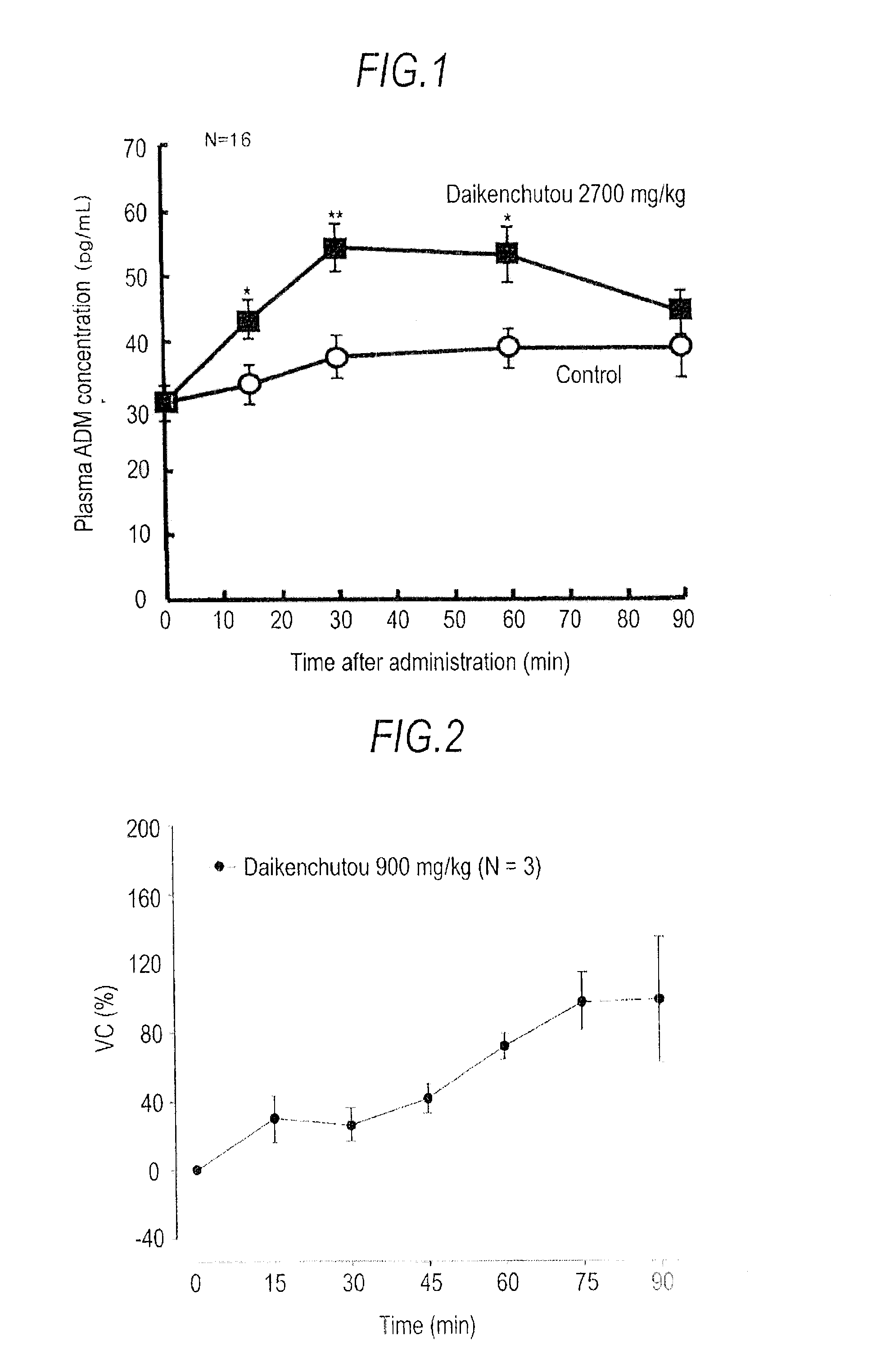
[0035]Adrenomedullin Production-Enhancing Effect Of Daikenchutou:
[0036]Male SD rats (8 to 10 weeks of age, body weight 300 to 400 g) were used (n=16). Saccharum granorum was added to distilled water to a concentration of 480 mg / mL to prepare an aqueous solution of it. To this aqueous solution of Saccharum granorum, a powdered extract of Daikenchutou was added to a concentration of 60 mg / mL, by weighing immediately at the time of use, and the mixture was homogeneously dispersed by stirring for 30 minutes at room temperature for use as a test specimen. A 5 mL / kg portion of the test specimen, which had been kept warm at 37° C., was administered into the duodenum through a cannula, and blood was collected from the portal vein at 0, 15, 30, 60, and 90 minutes after administration. A control group was administered distilled water in the same fashion. Approximately 5 mL of the portal vein blood was inserted into a 15-mL centrifuge tube made of polypropylene (PP), in which an ethylenediamin...
example 2
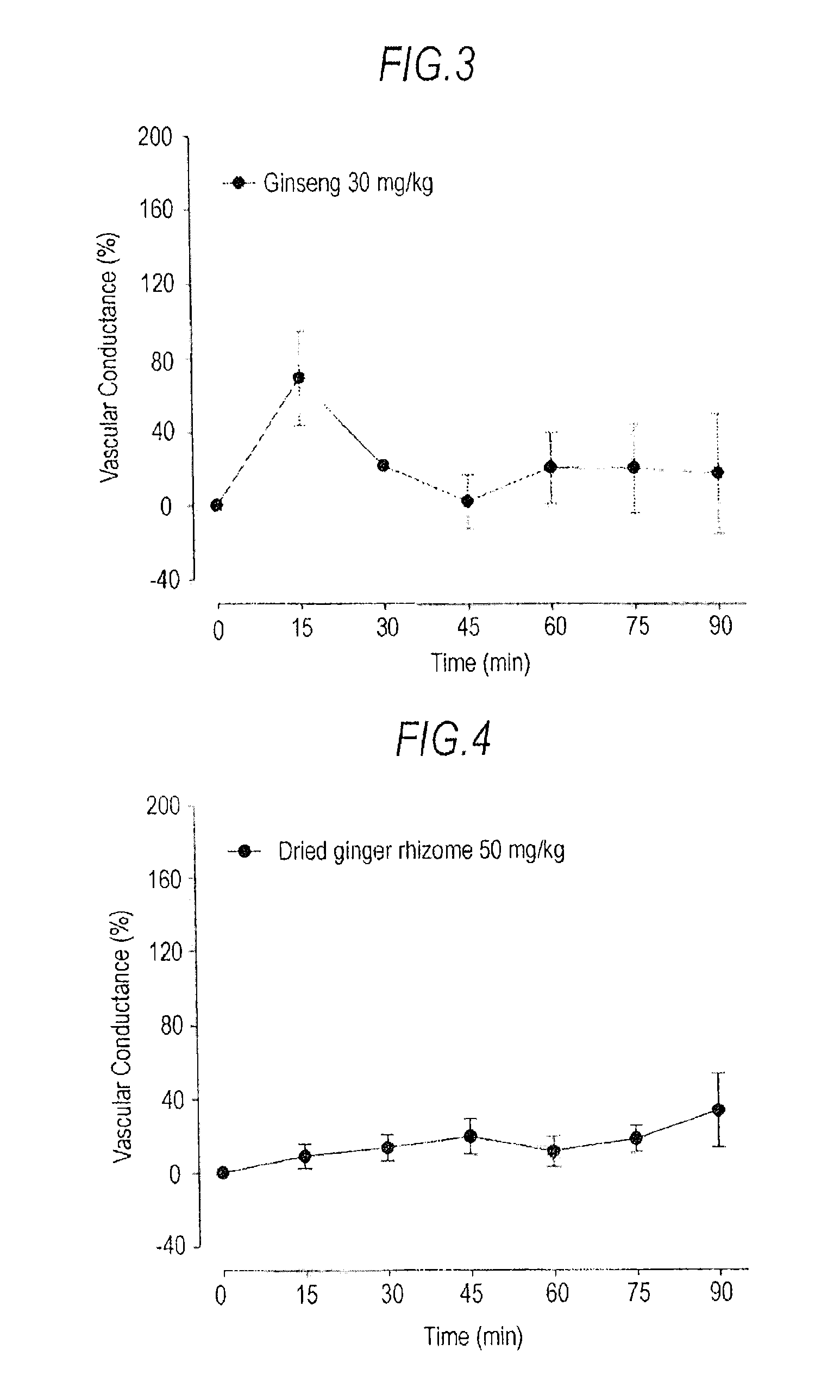
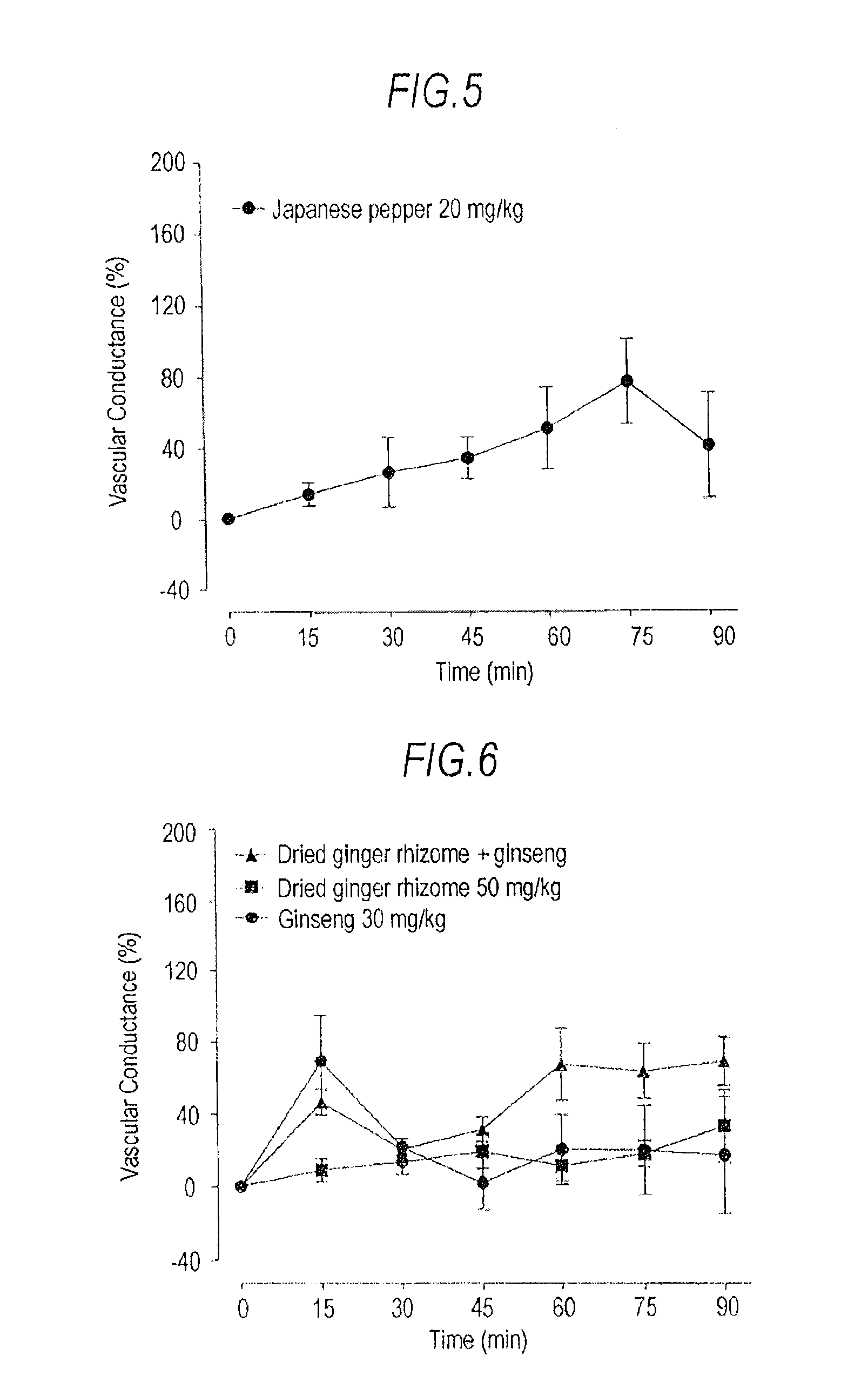
[0040]Blood Flow-Increasing Effects Of Daikenchutou And Its Constituent Crude Drugs:
[0041]Male SD rats (9 to 11 weeks of age, body weight 260 to 350 g) were used (n=3 or 6). Saccharum granorum and a powdered extract of Daikenchutou were suspended in distilled water to obtain a concentration of 160 mg / mL of Saccharum granorum and to a concentration of 20 mg / mL for the powdered extract of Daikenchutou, and the suspension was used as a test specimen (Daikenchutou). Furthermore, test specimens respectively containing the individual crude drugs were prepared by adding the ginseng extract powder, dried ginger rhizome extract powder, and Japanese pepper extract powder to distilled water to obtain concentrations of 6 mg / mL, 10 mg / mL, and 4 mg / mL, respectively. Furthermore, test specimens containing two crude drugs among the aforementioned three crude drugs in combination were prepared by addition to distilled water to obtain concentrations of 6 mg / mL for the ginseng extract powder and 10 mg...
example 3
[0044]Effect Of ADM Antagonist Pretreatment On The Blood Flow-Increasing Effect Of Daikenchutou:
[0045]Male SD rats were divided into two groups: a Daikenchutou-treated group and an ADM antagonist-pretreated group (n=7 or 8). A solution with a 160 mg / mL concentration of Saccharum granorum was prepared using distilled water, and powdered Daikenchutou extract was weighed at the time of use to obtain a concentration of 20 mg / mL and homogeneously dispersed in the same solution by stirring at room temperature for 30 minutes or longer to obtain Daikenchutou (TJ-100). This was administered into the colon in the Daikenchutou-treated group in an amount of 900 mg / 5 mL / kg. On the other hand, for the ADM antagonist-pretreated group, a human ADM antagonist (h. ADM22-52, 4302-v, manufactured by Peptide Institute, Inc.) was dissolved in physiological saline at 30 μmol / L, and the solution was intravenously administered through a cannula placed under anesthesia, at a dose of 30 nmol / l mL / kg, and 15 m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com