Vaccine Compositions Comprising a Saponin Adjuvant
a technology of adjuvant and vaccine, which is applied in the direction of dsdna viruses, immunological disorders, antibody medical ingredients, etc., can solve the problems of high toxicity of these molecules, preventing their use in vaccine formulations and affecting the quality of life of patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example i
Immunological Read-Out Methods
I.1. Mice Methods
I.1.1. Hemagglutination Inhibition Test
Test Procedure
[0323]Anti-Hemagglutinin antibody titers to the three influenza virus strains were determined using the hemagglutination inhibition test (HI). The principle of the HI test is based on the ability of specific anti-influenza antibodies to inhibit hemagglutination of chicken red blood cells (RBC) by influenza virus hemagglutinin (HA). Heat inactivated sera were previously treated by Kaolin and chicken RBC to remove non-specific inhibitors. After pretreatment, two-fold dilutions of sera were incubated with 4 hemagglutination units of each influenza strain. Chicken red blood cells were then added and the inhibition of agglutination was scored. The titers were expressed as the reciprocal of the highest dilution of serum that completely inhibited hemagglutination. As the first dilution of sera was 1:20, an undetectable level was scored as a titer equal to 10.
[0324]Statist...
example ii
Preparation of the MPL / QS21 Liposomal Adjuvant
II.3 Preparation of MPL Liquid Suspension
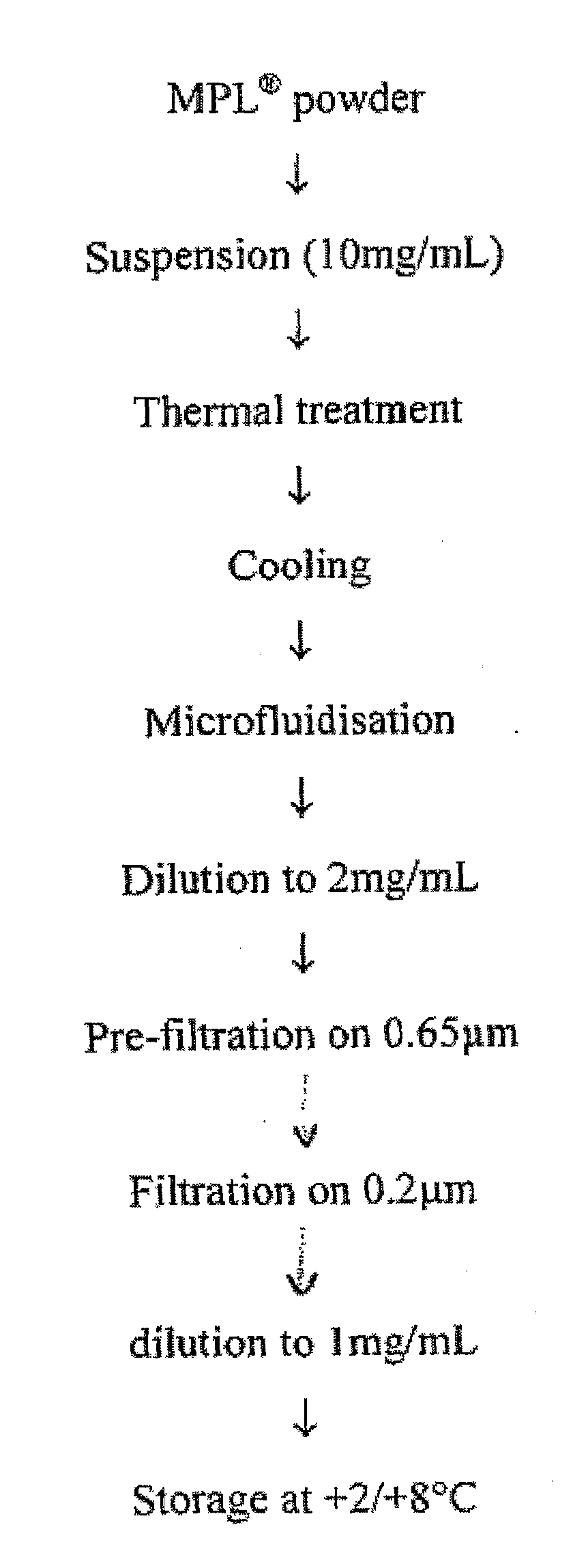
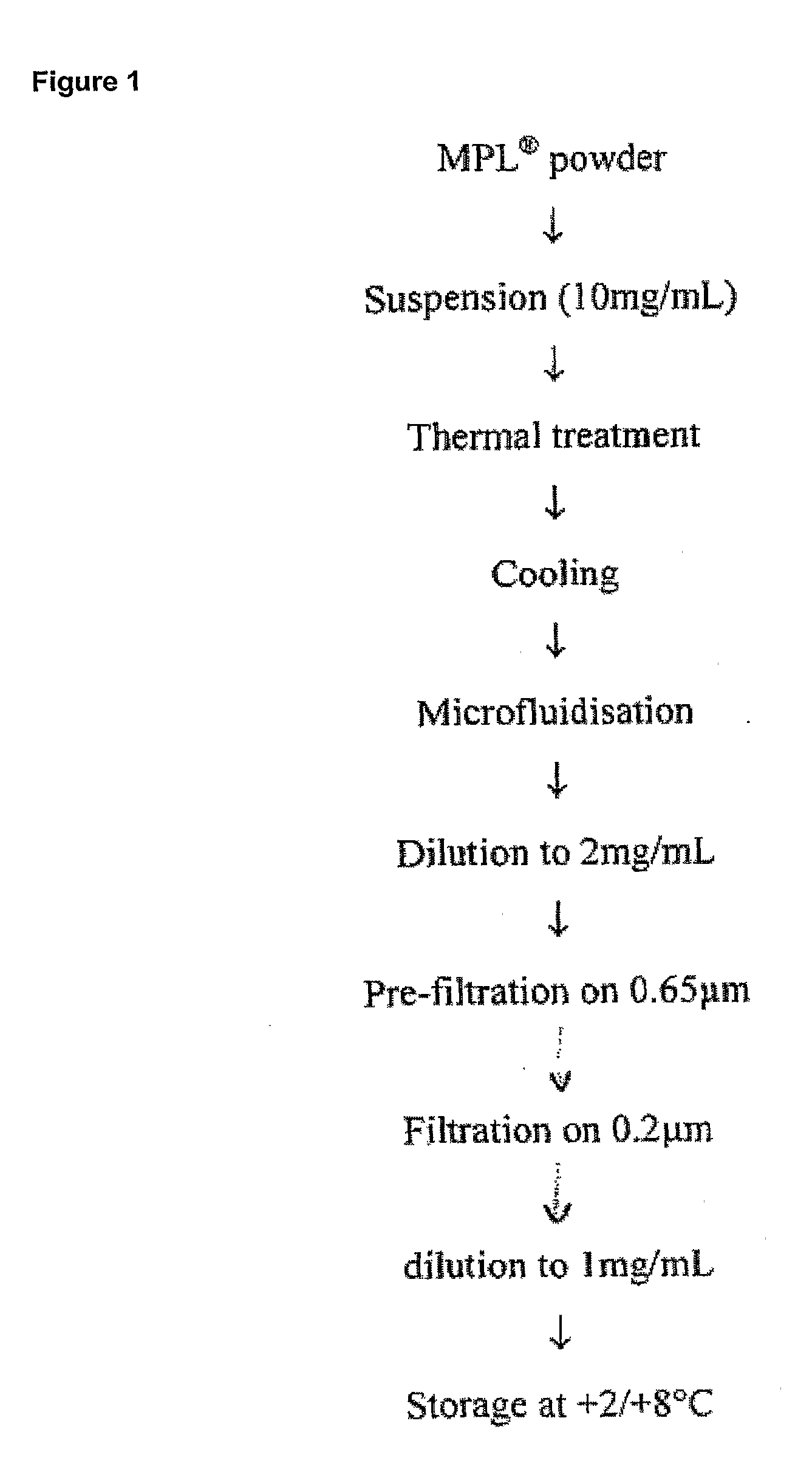
[0383]The MPL (as used throughout the document it is an abbreviation for 3D-MPL, i.e. 3-O-deacylated monophosphoryl lipid A) liquid bulk is prepared from 3D-MPL lyophilized powder. MPL liquid bulk is a stable concentrated (around 1 mg / ml) aqueous dispersion of the raw material, which is ready-to-use for vaccine or adjuvant formulation. A schematic representation of the preparation process is given in FIG. 1.
[0384]For a maximum batch size of 12 g, MPL liquid bulk preparation is carried over in sterile glass containers. The dispersion of MPL consists of the following steps:[0385]suspend the MPL powder in water for injection[0386]desaggregate any big aggregates by heating (thermal treatment)[0387]reduce the particle size between 100 nm and 200 nm by microfluidization[0388]prefilter the preparation on a Sartoclean Pre-filter unit, 0.8 / 0.65 μm[0389]sterile filter the preparation at room temperature (Sa...
example iii
Pre-Clinical Evaluation of Adjuvanted and Unadjuvanted Influenza Vaccines in Ferrets
III.1. Rationale and Objectives
[0398]Influenza infection in the ferret model closely mimics human influenza, with regards both to the sensitivity to infection and the clinical response.
[0399]The ferret is extremely sensitive to infection with both influenza A and B viruses without prior adaptation of viral strains. Therefore, it provides an excellent model system for studies of protection conferred by administered influenza vaccines.
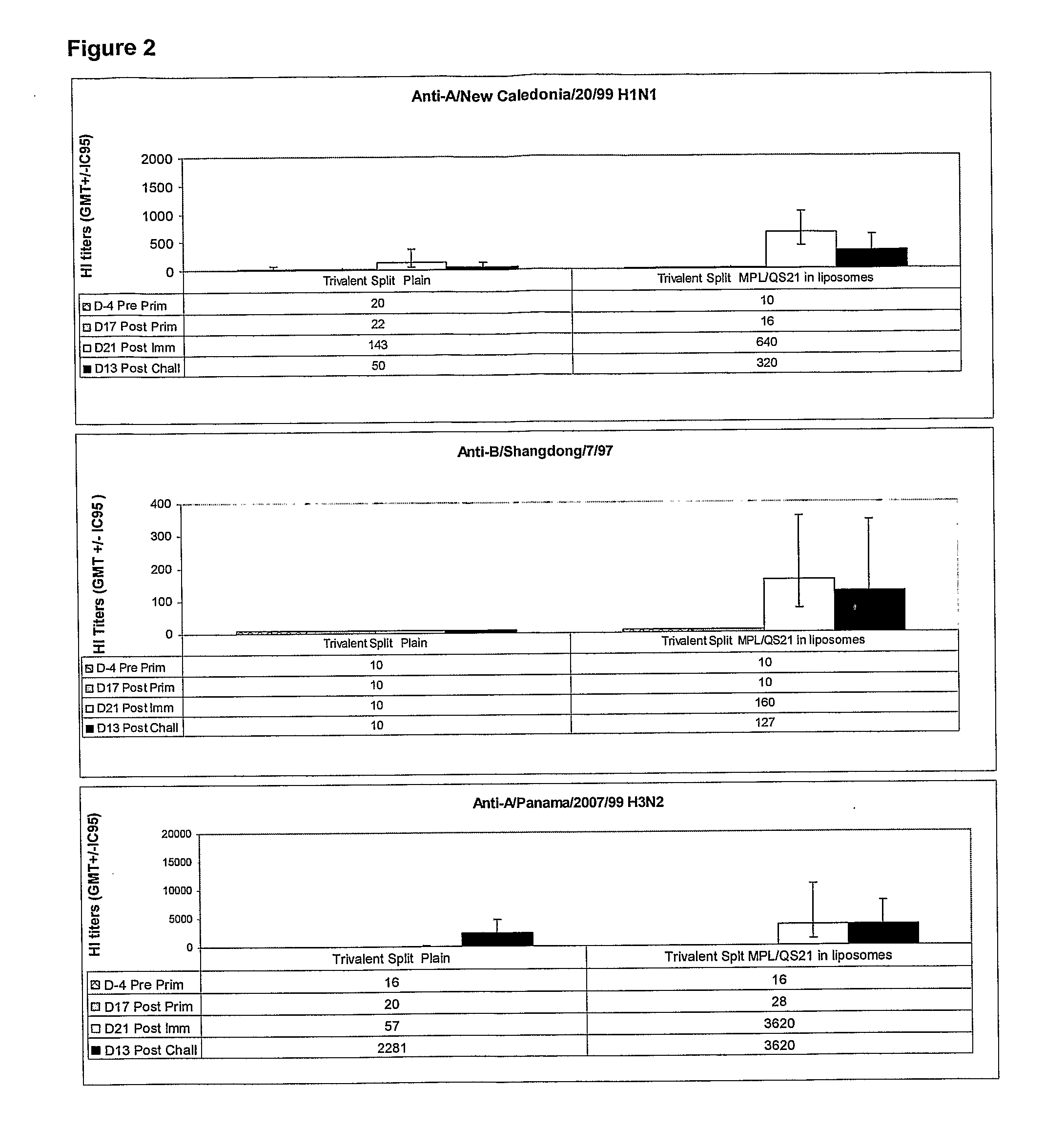
[0400]This study investigated the efficacy of various Trivalent Split vaccines, adjuvanted or not, to reduce disease symptoms (body temperature) and viral shedding in nasal secretions of ferrets challenged with homologous strains.
[0401]The objective of this experiment was to demonstrate the efficacy of an adjuvanted influenza vaccine compared to the plain (un-adjuvanted) vaccine.
[0402]The end-points were:
1) Primary end-point: reduction of viral shedding in nasal washes af...
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com