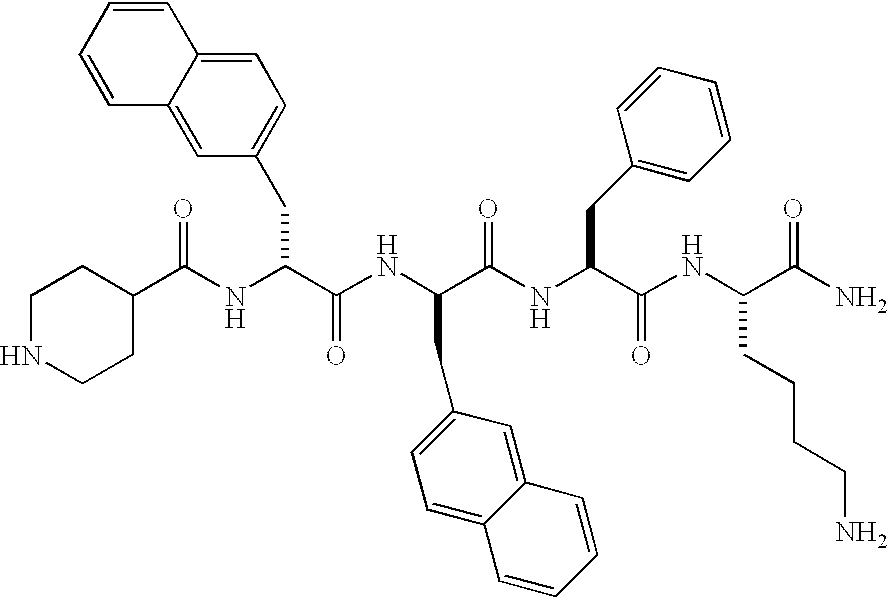
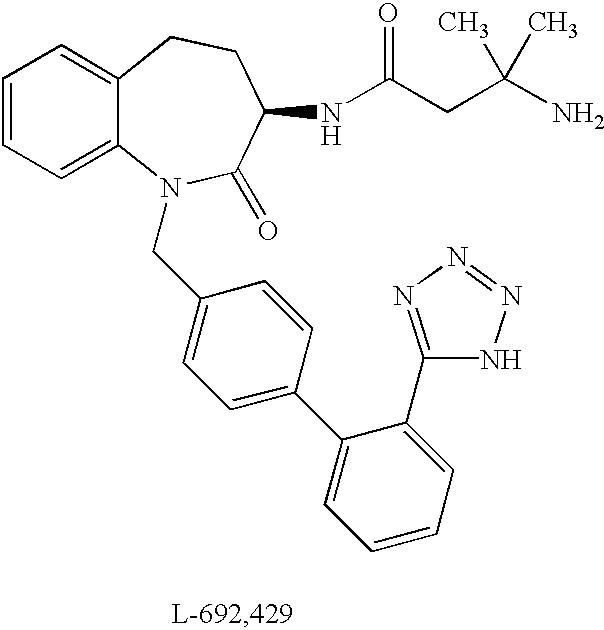
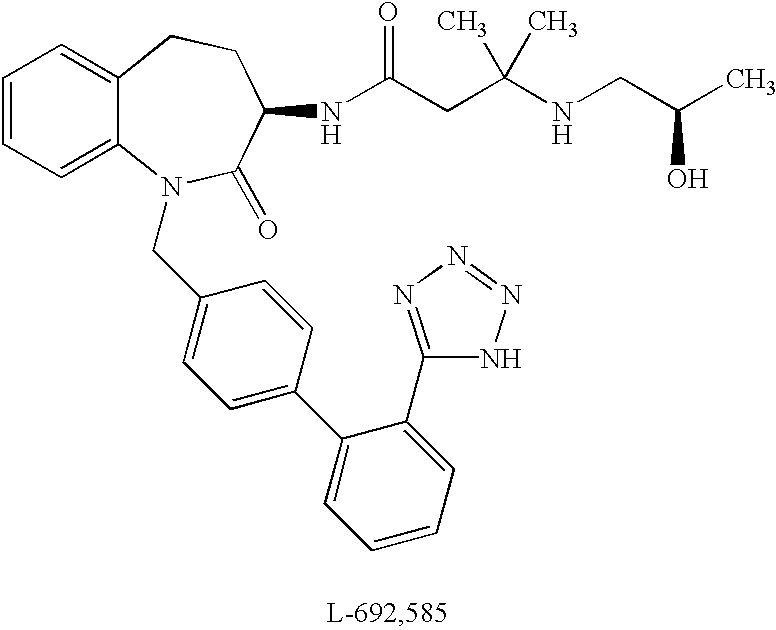
Low molecular weight peptidomimetic growth hormone secretagogues
a growth hormone and low molecular weight technology, applied in the field of new drugs, can solve the problems of peptides, lack of specificity for gh release, oral availability and specificity, etc., and achieve the effect of less effect on blood glucose and reduced effect on insulin sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0391] 48
Method A
[0392] Step A: (N-.epsilon.-BOC)Lys-(Am-resin)
[0393] FMOC-Am-resin (10 g, 0.50 mmol / g, 5.0 mmol) was deblocked by agitating with 20% piperidine in DMA for 15 min followed by successive washes with DMA (5.times.) and DCM (1.times.). The resin gave a positive test with ninhydrin. A solution of 9.37 g (20.0 mmol) of FMOC-(N-.epsilon.-BOC)-L-Lysine, 8.85 g (20.0 mmol) of BOP, and 3.31 mL (30.0 mmol) of NMM in 30 mL of DMA was added and the solution agitated for 1 h. The resin was washed (5.times.) with DMA and shown to give a negative ninhydrin test. The N-.alpha.-FMOC protecting group was removed with 20% piperidine in DMA for 15 min, followed by successive washings with DMA (5.times.) and DCM (1.times.) to give (N-.epsilon.-BOC)Lys-(Am-r-esin).
[0394] Step B: Phe-(N-.epsilon.-BOC)Lys-(Am-resin)
[0395] A solution of FMOC-L-Phenylalanine (7.75 g, 20.0 mmol) and BOP (8.85 g, 20.0 mmol) in 50 mL of DMA / DCM (1:1) was preactivated for 10 min and added to the (N-.epsilon.-BOC)...
example 2
[0432] 49
[0433] FMOC-D.beta.Nal-D.beta.Nal-Phe-(N-.epsilon.-BOC)Lys-(Am-resin) (1.0 g, 0.32 mmol), from Example 1, Method A, step D, was swelled with DCM for 15 min, deblocked with 20% piperidine in DMA for 15 min, and washed with DMA (5.times.) and DCM (1.times.) to give D.beta.Nal-D.beta.Nal-Phe-(N-.e-psilon.-BOC)Lys-(Am-resin), displaying a positive ninhydrin test. A preactivated solution of N-BOC-4-aminobutyric acid (403 mg, 1.98 mmol), 875 mg (1.98 mmol) of BOP, and 0.33 mL (2.97 mmol) of NMM in 15 mL of DMA / DCM (1:1) was added. After agitation for 1 h, the resin was washed with DMA (5.times., ninhydrin negative), DCM (3.times.), MeOH (2.times.) and dried in vacuo. The dry resin was suspended in 10 mL of TFA and 0.50 mL of triethylsilane added. The mixture was agitated for 1 h, concentrated in vacuo, and the resin washed with ether. The crude peptide was recovered from the resin by washing with 10% aq HOAc, followed by acetonitrile. The combined filtrates were lyophilized to gi...
example 3
[0434] 50
[0435] Step A: BOC-4-(N-Methylamino)butyric Acid
[0436] To a 0.degree. C. solution of 5.00 g (24.6 mmol) of BOC-4-aminobutyric acid in 75 mL of dry THF, was added 12.2 mL (197 mmol) of methyl iodide followed by 2.95 g (73.8 mmol, 60% dispersion in mineral oil) of sodium hydride, portionwise. The reaction was rapidly stirred at room temperature for 12 h and quenched by the careful addition of water. The mixture was partitioned between ether and water, and the organic phase extracted with 1 N aq sodium bicarbonate. The combined aqueous phases were chilled and acidified to pH 3 with 1 N sodium hydrogen sulfate, then extracted with two portions of ethyl acetate. The combined organics were washed successively with water, 5% aq sodium thiosulfate, water, brine, and then dried over anhydrous magnesium sulfate. Concentration in vacuo afforded 5.00 g (94%) of BOC-4-(N-methylamino)buty-ric acid. .sup.1H NMR: (300 MHz, CDCl.sub.3) .delta. 3.28 (2H, bt, J=7 Hz), 2.84 (3H, s), 2.36 (2H, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight gain | aaaaa | aaaaa |
| weight gain | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com