Oridonin clathrate compound, and medicinal prepn. thereof
A technology of Rubescensin A and clathrate, which is applied in the direction of medical preparations containing active ingredients, non-active ingredients of polymer compounds, drug combinations, etc., and can solve the problem of low drug loading and encapsulation efficiency and complicated process , can not be applied and other problems, to achieve the effect of improving solubility and bioavailability, simple process and low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Example 1 Preparation of Oridonin Cyclodextrin Inclusion Compound
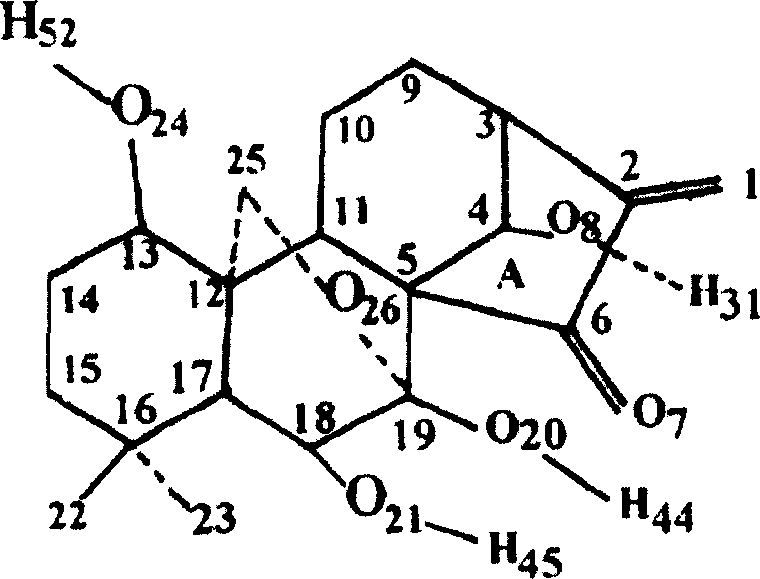
[0052] Oridonin A
10.0g
2,6-Dimethyl-β-cyclodextrin
20.0g
20ml
Water for Injection
300ml
[0053] The preparation method of cyclodextrin inclusion compound is as follows:
[0054] Weigh 10.0g of Rubescensine A, dissolve in 20ml of ethanol; weigh 20.0g of 2,6-dimethyl-β-cyclodextrin, dissolve in 300ml of water for injection (deionized water can also be used) . After the two were mixed, the reaction was stirred for 4 hours, the temperature was controlled at about 60° C., and the reaction was stirred for 4 hours. Let it stand for 3 days, filter, and dry it.
[0055] Solubility test results show that the solubility of the clathrate is more than 10 times higher than that of oridonin. Stability after exposure to light is also significantly improved.
Embodiment 2
[0056] Example 2 Preparation of Oridonin A Cyclodextrin Inclusion Compound
[0057] Oridonin A
[0058] Refer to Example 1 for the preparation process.
Embodiment 3
[0059] Example 3 Preparation of Rubescensin A Cyclodextrin Inclusion Compound
[0060] Oridonin A
[0061] Refer to Example 1 for the preparation process.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com