Curcumin liposome and its method for preparing freeze-dried powder injection
A technology of freeze-dried powder injection and liposome, which is applied in the field of preparation of curcumin liposome and its freeze-dried powder injection, can solve the problems of instability, poor water solubility of curcumin, difficulty in making injections, etc., and achieve improved stability Sex, increase stability and clinical safety, and reduce the effect of unsafe factors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
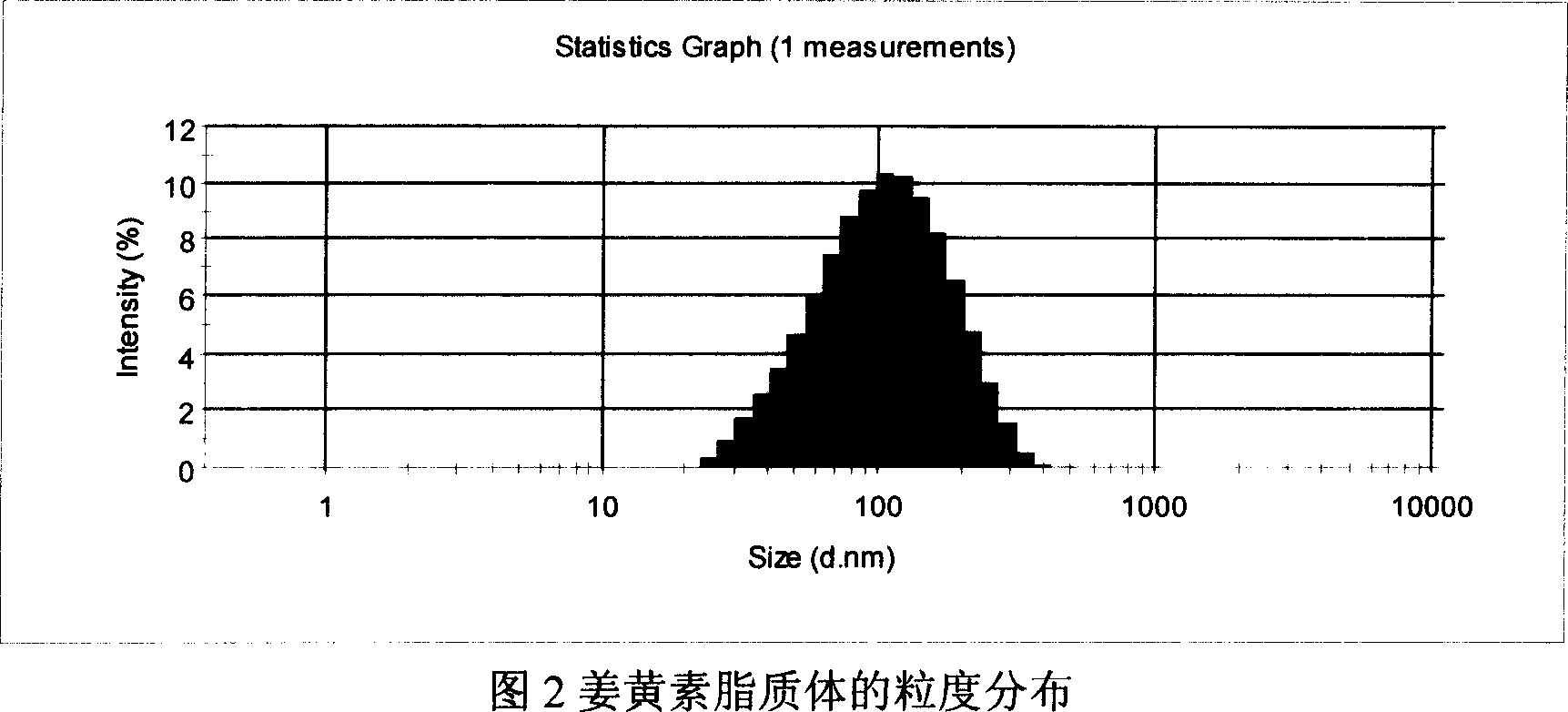
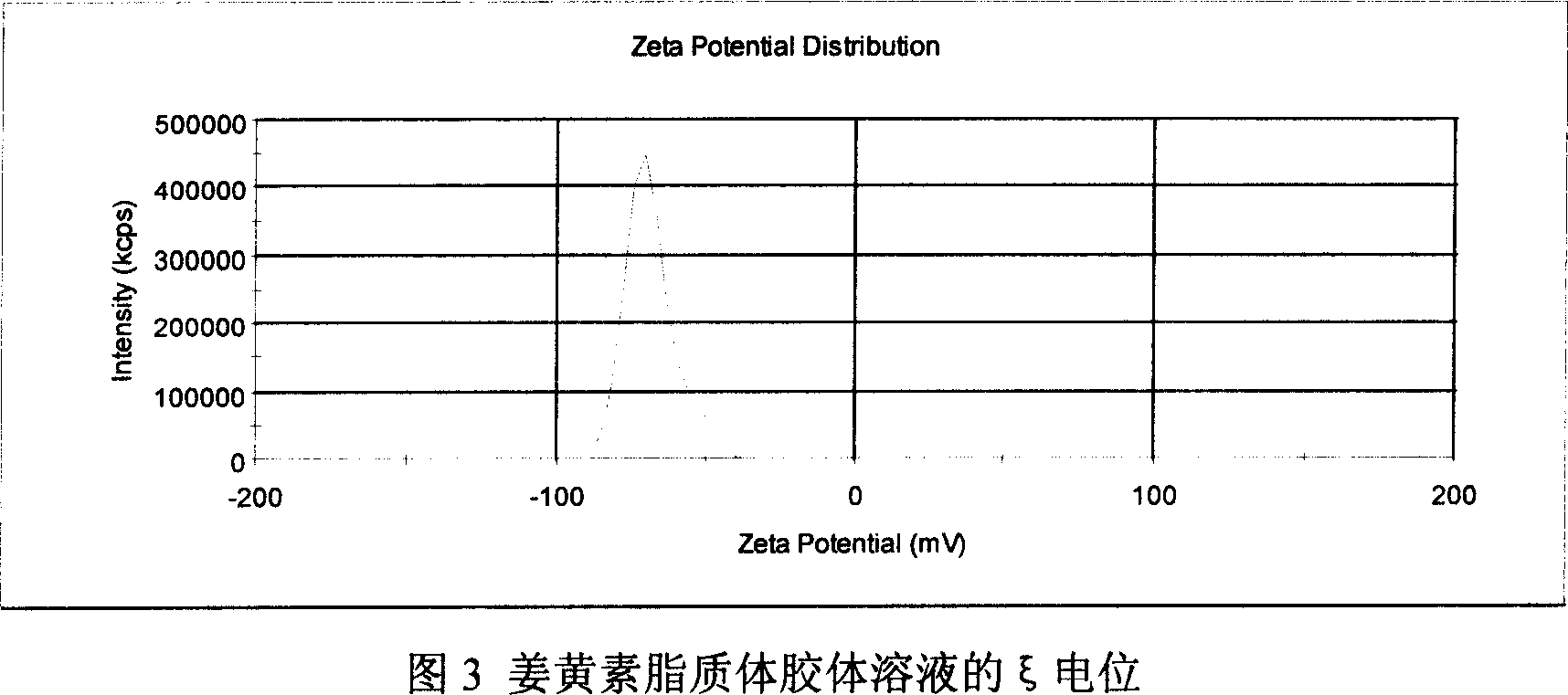
[0042]Dissolve 60 mg of curcumin, 0.6 g of hydrogenated soybean lecithin (purity > 95%), and 60 mg of DSPG in a mixed solvent of chloroform / methanol (1:1) to completely dissolve into a clear solution, and dry it under reduced pressure on a constant temperature water bath at 40°C to form a film , placed in a vacuum desiccator for further drying overnight. Add sodium succinate solution (pH6.5) with sucrose to dissolve the membrane at 55°C until multilamellar liposomes (MLV) are formed, and high-pressure homogenization (Niro homogenizer model, NS1001L) to reduce the particle size (1500bar, 5 times) that is to say. After aseptic filtration (the pore size of the membrane filter is 0.22 μm), it is divided into vials and freeze-dried to obtain curcumin liposome freeze-dried powder. After being stored at 40° C. for 3 months, the content of curcumin was 93.2%. Adding water for injection to reconstitute the liposome had an encapsulation efficiency of 89.7% and an average particle diame...
Embodiment 2
[0044] Dissolve 60mg of curcumin, 1.2g of egg yolk lecithin (purity > 95%), and 120mg of DOPG in a mixed solvent of chloroform / methanol (1:1), place in a constant temperature water bath at 35°C to dry under reduced pressure to form a film, and place in a vacuum desiccator Further dry overnight. Add PBS solution (pH6.0) with trehalose to dissolve the membrane at 55°C until multilamellar liposomes (MLV) are formed, and high-pressure homogenization (Niro homogenizer model, NS1001L) reduces the particle size (1500bar , 5 times) to get. After aseptic filtration (the pore size of the membrane filter is 0.22 μm), it is divided into vials and freeze-dried to obtain curcumin liposome freeze-dried powder. After being stored at 40°C for 3 months, the content of curcumin was 94.3%, and the encapsulation efficiency of the liposome reconstituted by adding water for injection was 92.5%, and the average particle diameter was 140.2nm.
Embodiment 3
[0046] Dissolve 60 mg of curcumin, 1.8 g of egg yolk lecithin (purity > 95%), and 180 mg of DSPG in a mixed solvent of chloroform / methanol (1:1) to completely dissolve into a clear solution, and place it in a constant temperature water bath at 38°C to dry under reduced pressure to form a film , placed in a vacuum desiccator for further drying overnight. Add sodium citrate solution (pH5.5) with mannitol to dissolve the membrane at 55°C until multilamellar liposomes (MLV) are formed, and high-pressure homogenization (Niro homogenizer model, NS1001L) to reduce the particle size (1500bar, 5 times) that is to say. After aseptic filtration (the pore size of the membrane filter is 0.22 μm), it is divided into vials and freeze-dried to obtain curcumin liposome freeze-dried powder. After being stored at 40° C. for 3 months, the content of curcumin was 95.6%, and the encapsulation rate of the liposome obtained by adding water for injection to reconstitute was 94.7%, and the average par...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com