Long-acting reconbinant tissue factor channel inhibitor and preparing method thereof
A technology of tissue factor and inhibitors, which is applied in the field of functional determination and long-acting recombinant tissue factor pathway inhibitors, can solve the problems of short half-life and achieve the effects of prolonged half-life, simple preparation process and high-efficiency anticoagulation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Embodiment 1 The docking and analysis of TFPI and LRP on a computer workstation
[0018] Using Insight II software, TFPI and LRP were docked on a computer workstation, and the 269, 282, 288 and 289 lysines at the carboxy terminus of TFPI were found to be the key sites for the interaction between the two.
Embodiment 2
[0019] Design, preparation and characterization of embodiment 2 LTFPI
[0020] (1) Cloning and transformation of LTFPI gene and construction of prokaryotic expression plasmid rLTFPI-pET28
[0021] The design method of LTFPI is as follows: replace the lysine at 269, 282, 288 and 289 positions of the carboxy terminus of TFPI with alanine. The sequence was obtained by PCR point mutation, recombined with pUC19, positive clones were screened by enzymatic hydrolysis, and the nucleotide sequence analysis confirmed that the gene sequence was correct. Then the LTFPI gene was cut out and recombined with the prokaryotic expression vector pET28 to form the prokaryotic expression plasmid rLTFPI-pET28. Transform Escherichia coli JM109, extract the plasmid, identify with the corresponding restriction endonuclease, obtain characteristic fragments, and confirm that positive clones were obtained.
[0022] The method of obtaining the LTFPI gene is as follows:
[0023] Using the overlap extens...
Embodiment 3
[0062] Embodiment 3 LTFPI half-life determination
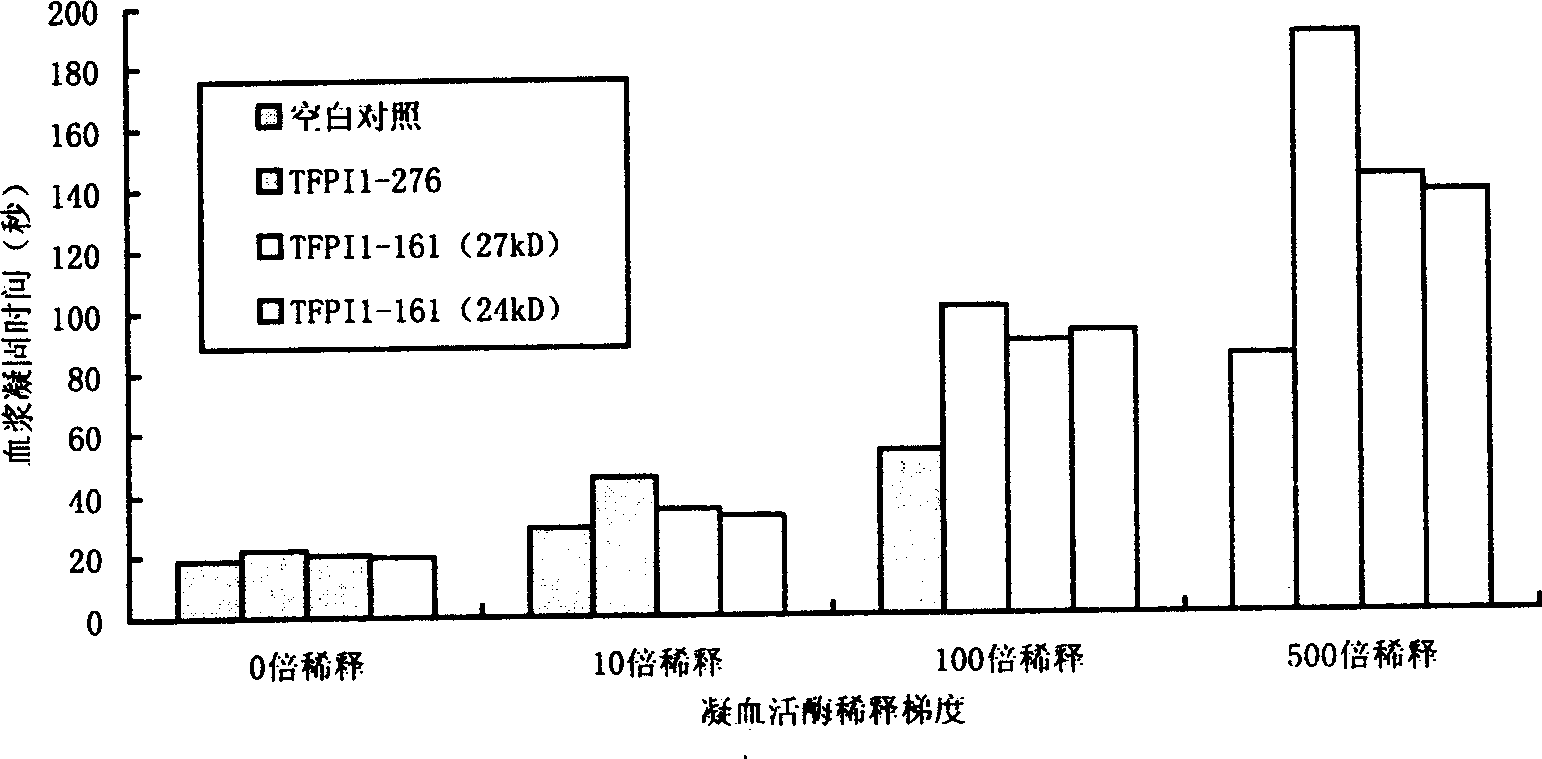
[0063] The half-life of TFPI in rats was measured by dPT method: rats were anesthetized by intraperitoneal injection of pentobarbital sodium (40 mg / kg), administered via femoral vein (1 mg / kg), intubated in the common carotid artery, and blood was collected at different times after administration. Anticoagulate with 109mM sodium citrate 1:9, centrifuge at 4°C for 15min, 4000r / min, absorb the upper layer of plasma, and measure dPT.
[0064] Dilute the standard solution of thromboplastin 500 times with normal saline, and this is the working solution of thromboplastin. Take 100 μL plasma, add 100 μL thromboplastin working solution, incubate at 37°C for 1 min, add 10 μL 250mmol / LCaCl 2 solution, start timing the plasma coagulation time. The average value of 3 experiments was recorded as the experimental result.
[0065] Taking wild-type TFPI as the control group, observe the half-life of LTFPI (K282A) and LTFPI (K269A, K282A, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com