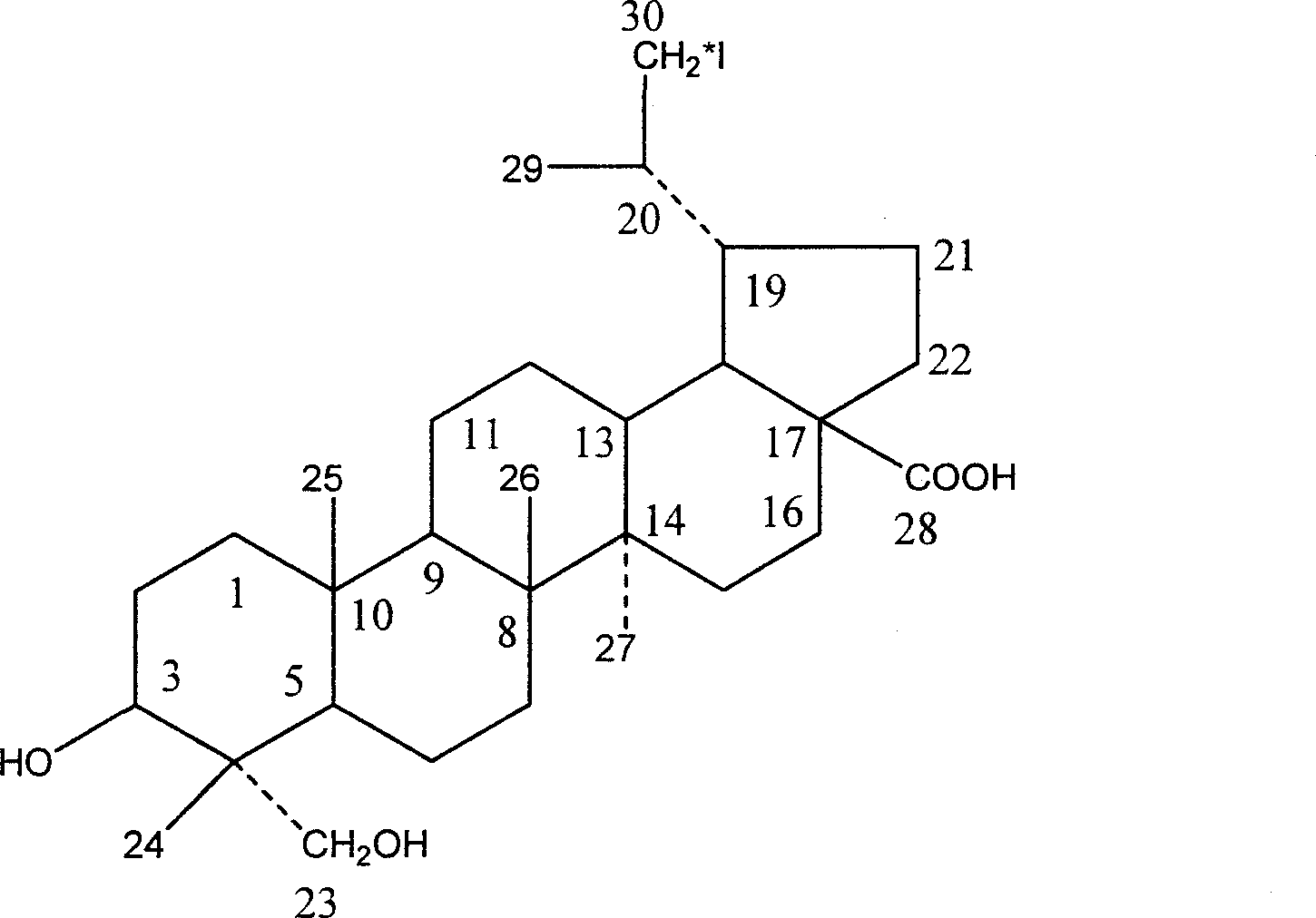
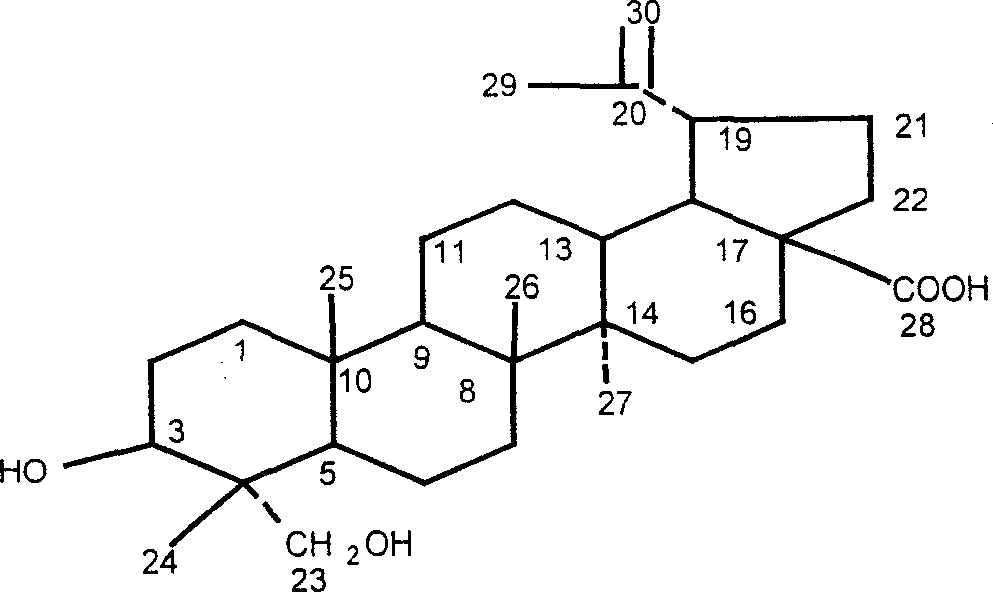
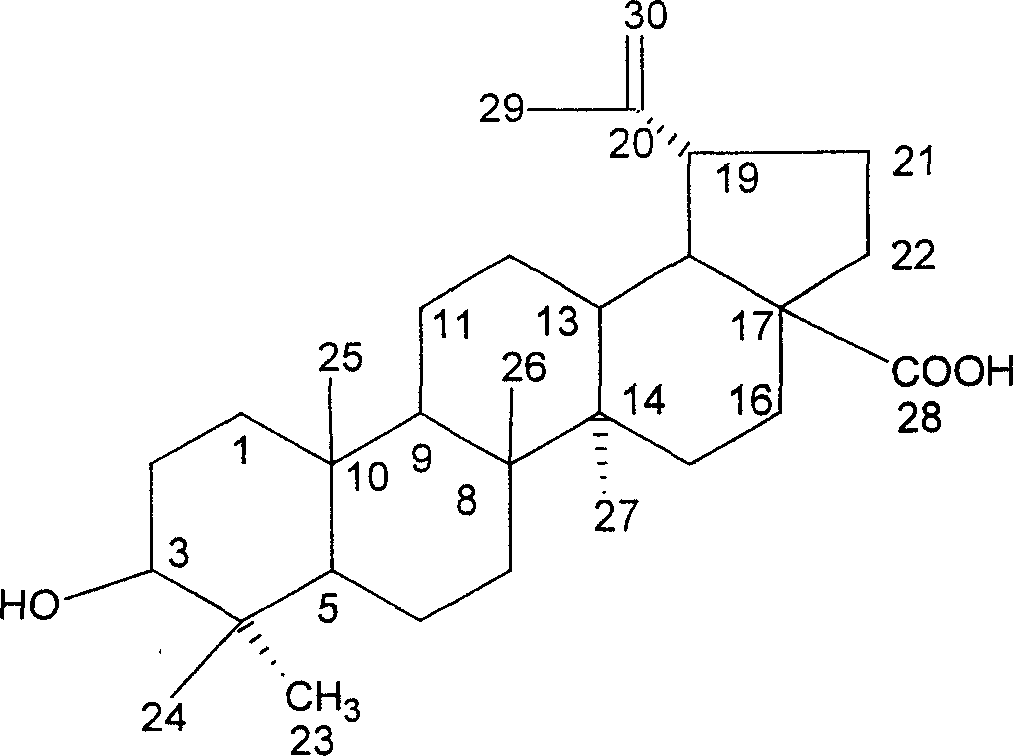
Iodine (I)-23-hydroxyl betulinic acid, rpeparation method and application thereof
A betulinic acid and hydroxyl technology, applied in the fields of oncology, pharmaceutical preparations, and nuclear medicine, can solve problems such as no foreign reports, unclear molecular mechanism, etc., and achieve a simple preparation process, good stability, and strong anti-tumor pharmacological effects. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1: * Preparation of I-23-Hydroxybetulinic Acid
[0037]
[0038] A: Dissolve 20 μg of 23-hydroxybetulinic acid in 20 μL of ethanol, add 20 μL of 4mol / L hydrochloric acid solution, add 20 μL of 3% hydrogen peroxide, and add 1 mCiNaI * , vortexed for 1min to mix well, and placed at 40°C for 15min; adding 0.01mol / L sodium metabisulfite to terminate the reaction. Adjust the pH to 7 with ammonia water.
[0039] B: Add 2 mL of water to A, extract with ethyl acetate (5 mL x 3 times), collect the organic phase, and dry it with nitrogen in a water bath at 60°C.
Embodiment 2
[0040] Example 2: * Identification of I-23-hydroxybetulinic acid
[0041] Determination by thin layer chromatography and high performance liquid chromatography.
[0042] A: Thin-layer chromatography: silica gel paper as support, developing solvent as dichloromethane / methanol=9 / 1, Rf=0.5-0.7. After sample application, when the development is completed, the markers are detected as yellow spots at Rf=0.5-0.7 under ultraviolet light; the silica gel paper is cut into ten sections, and the radioactive count is measured by a gamma counter, and the radioactivity is concentrated at Rf=0.5-0.7. Free iodine is at Rf = 0-0.1.
[0043] B: HPLC: BIORAD HPLC, YWG C18 reverse phase column, acetonitrile / water=6 / 4 (containing 0.1% glacial acetic acid), flow rate 1mL / min, detect with ultraviolet detector (wavelength 254nm) and radioactive detector simultaneously, The retention time of free iodine is about 1.3min, * The retention time of I-23-hydroxy betulinic acid is about 5min.
Embodiment 3
[0044] Example 3: 131 Stability of I-23-hydroxybetulinic acid
[0045] Get the obtained by blowing dry under the item of embodiment 1 131 I-23-Hydroxybetulinic acid was dissolved in physiological saline and placed in a 4°C refrigerator at 37°C. After standing for 8 days, the radiochemical purity is still greater than 90%. It shows that the marker is stable at 4°C and 37°C, which can meet the requirements of routine clinical use.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com