188Re-labelled folic acid coupled cis-platinum magnetic albumin compound nano-particle as well as preparation method and application thereof
An albumin nanoparticle, 188re- technology, which is applied in the field of cisplatin magnetic albumin composite nanoparticle and its preparation, can solve the problems of uneven temperature rise, inability to measure and control temperature, etc., and achieve good stability and anti-tumor pharmacological effect. Strong, high-purity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Example 1: 188 Re-labeled folic acid-coupled cisplatin magnetic albumin composite nanoparticles ( 188 Preparation of Re-folate-CDDP / HAS MNP)
[0036] Add 0.1ml sodium gluconate solution (0.3mol / L), 0.1ml concentrated hydrochloric acid dissolved stannous chloride (10mg / ml), 0.04ml acetic acid buffer solution (pH value 5.0, 0.2mol / L) and 30mg of Folate-ADR-HAS-NP microspheres, shake well and place at room temperature for 1h; then add 188 ReO 4 Eluent 0.1ml, shake well, react at room temperature for 5h, and labeling is completed.
Embodiment 2
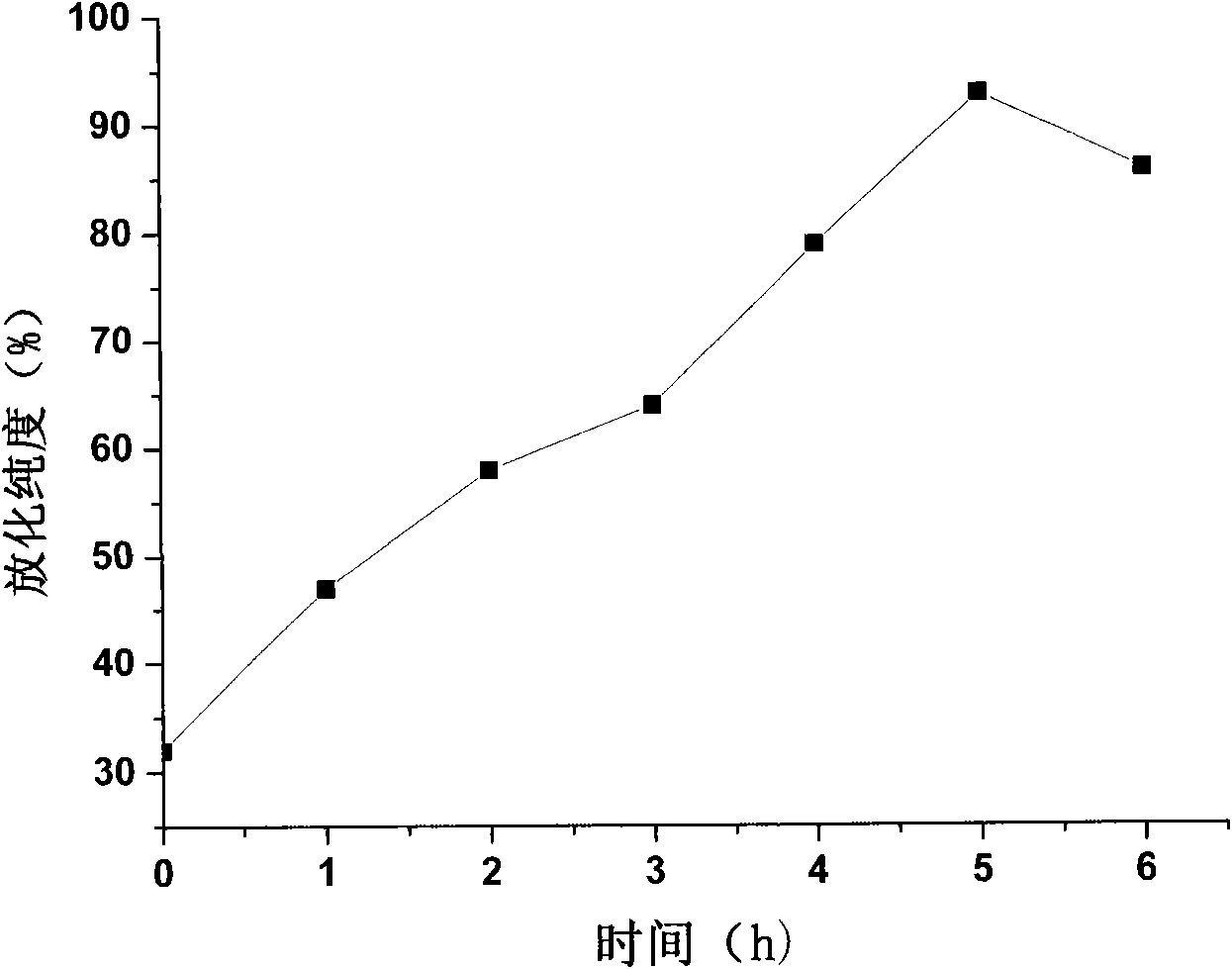
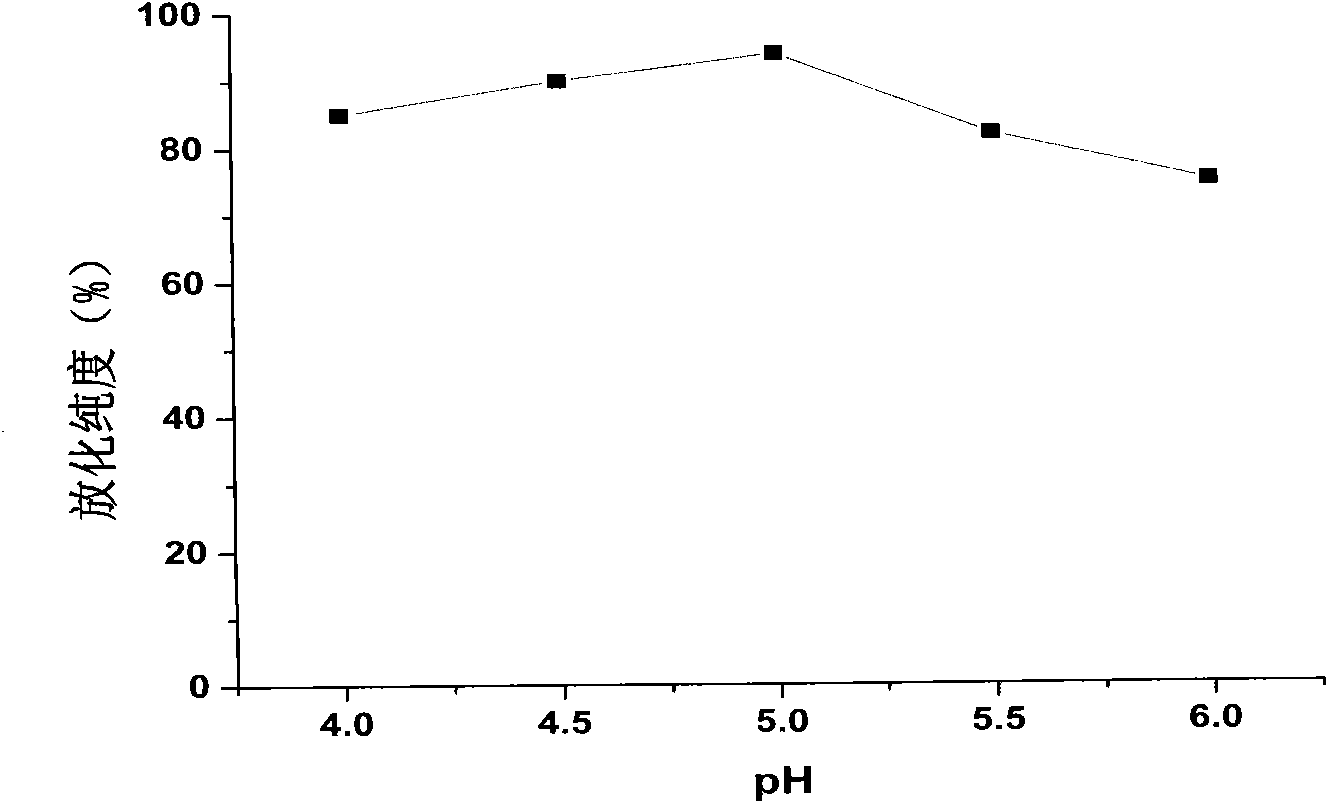
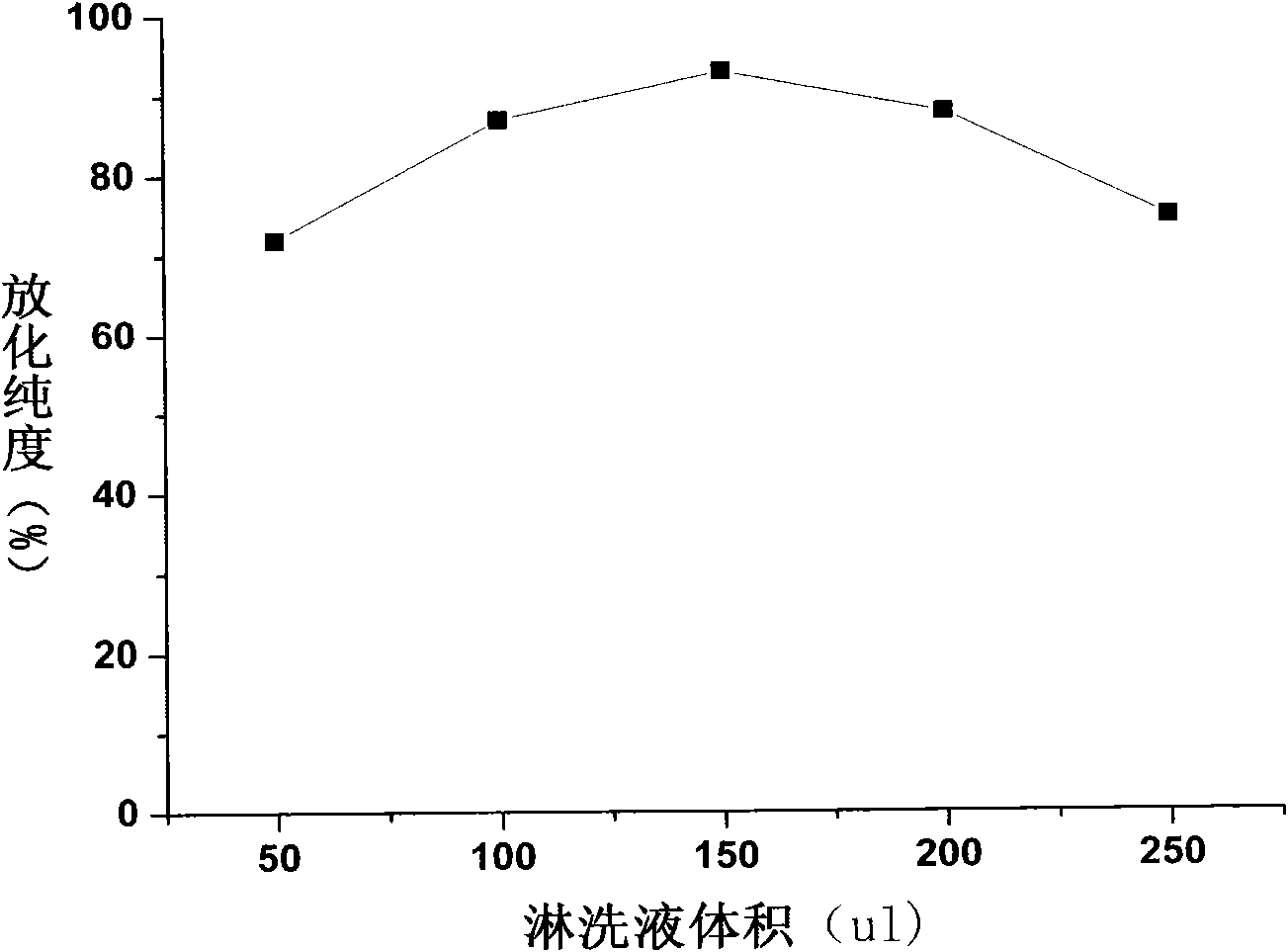
[0037] Example 2: 188 Determination of labeling efficiency of Re-labeled folic acid-coupled cisplatin magnetic albumin composite nanoparticles and optimization of labeling conditions
[0038] Thin-layer chromatography was used for analysis. The support was made of GF254 silica gel plate, which was baked in a 56°C oven for half an hour before use. Cut the silica gel plate into strips of about 0.5cm×10cm, mark the origin with a pencil, the origin is about 1cm away from the bottom edge of the test paper, and draw 10 horizontal lines with a pencil at equal distances, a total of 10 grids for later use; take the test tube and make For repeated tube detection, add developing agent, and the developing system is ethanol:ammonia:water (2:1:5v / v). Use a capillary pipette to take the labeled ependoff tube 188 Re-folate-CDDP / HAS MNP, apply the sample at the origin, dry it and run chromatography in the chromatography system. Take out the chromatographic paper and dry it, cut it into 10 ...
Embodiment 3
[0055] Example 3: 188 In Vitro Stability Observation of Re-Folate-ADR / HAS-NP
[0056] Take the composite magnetic nanoparticles marked under Example 1 and wash them twice with distilled water or normal saline, then disperse in 1 mL of calf serum, incubate with shaking at 37°C, at 0, 2, 4, 8, 12, 24, 48 , 72h sampling, and determine the radioactive count. The result is as Figure 5 As shown, it was found that the radiochemical purity was still greater than 80% after being placed at 37° C. for 72 hours. It shows that the marker is stable at 37°C, which can meet the requirements of routine clinical use.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com