Plasmid of recombinant immunotoxin IP 10-DT 390 aimed at activating Th1 cell, and its preparing method and use
An immunotoxin and recombinant plasmid technology, applied in recombinant DNA technology, drug combination, pharmaceutical formulations and other directions, can solve the problems of inability to effectively prevent disease recurrence, technical difficulties, toxic side effects, and unstable curative effects, and avoid the preparation process and It is difficult to standardize, reduce toxic and side effects, and is easy to industrialize.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
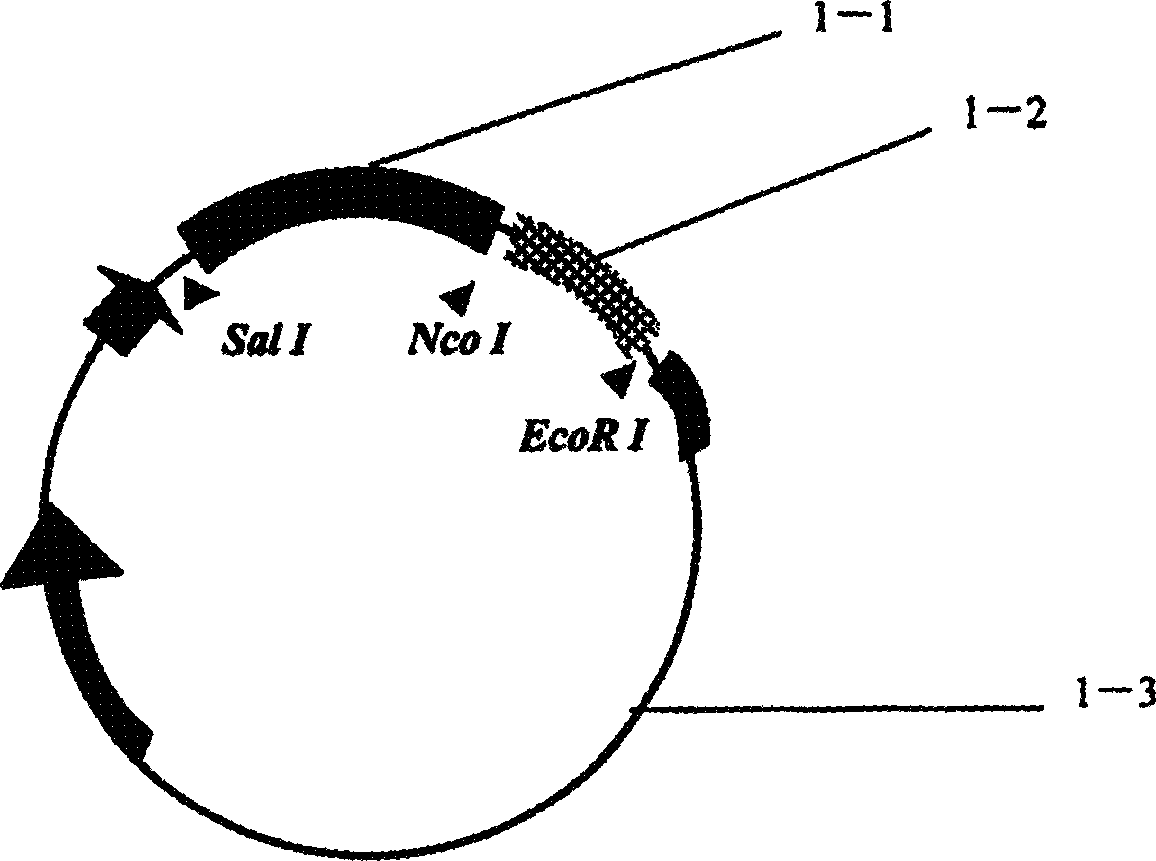
[0023] Example 1: Construction of recombinant immunotoxin eukaryotic expression plasmid
[0024] The vector used was the SRα eukaryotic plasmid containing DT390 (provided by PhD. Hu Huaizhong, University of Wisconsin, USA, Invitrogen Company), and the specific steps were as follows:
[0025] 1. Pretreatment of mouse IP10 gene (the following reagents were all purchased from Promega Company)
[0026] (1) Use Trizol reagent to extract mouse liver total RNA according to the following steps
[0027] 1) Mouse liver tissue 100mg.
[0028] 2) Add 1ml Trizol.
[0029] 3) homogenate.
[0030] The homogenate should be thorough, and then transferred to EP tubes. When the amount of tissue homogenate is > 100mg, it will be divided into 1ml / each EP tube.
[0031] 4) Inverted and mixed for 10 times, room temperature for 5 minutes.
[0032] 5) Add 1 / 5 volume of chloroform (0.2ml, must be 1 / 5 of the total volume).
[0033] 6) Inverted and mixed for 10 times, room temperature for 5 minutes...
Embodiment 2
[0079] Example 2: Determination of biological activity of recombinant immunotoxin IP10-DT390 in vitro
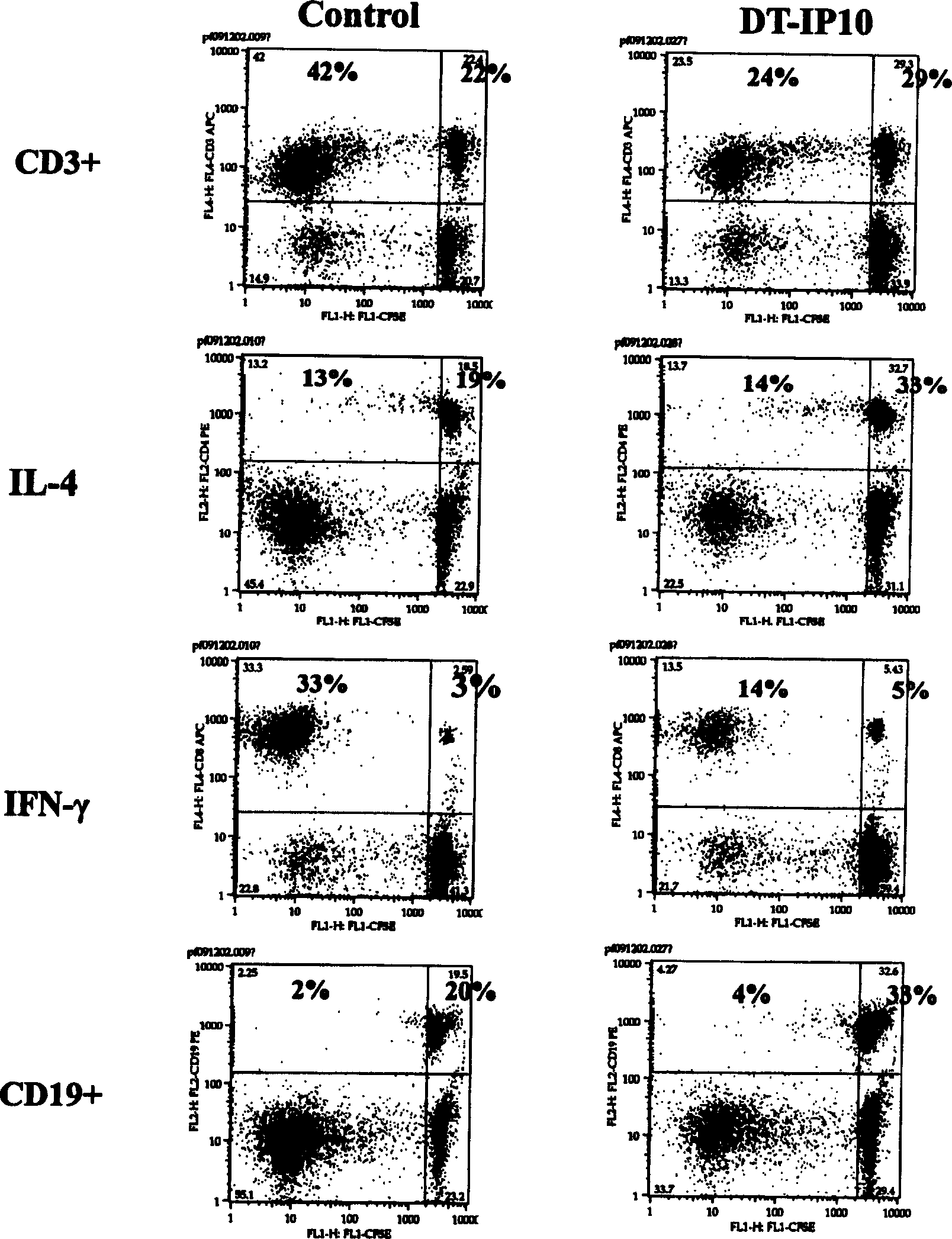
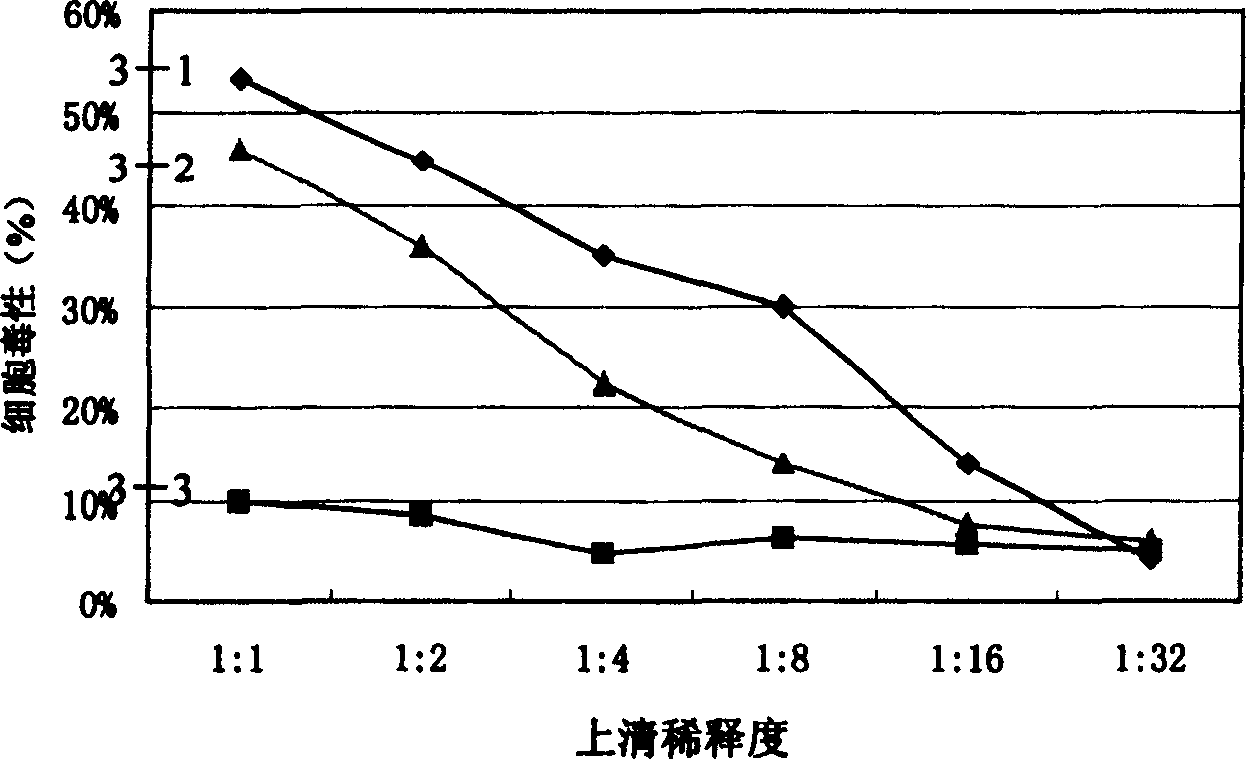
[0080] 1. Determination of cytotoxicity by flow cytometry:
[0081] (1) Take the mouse spleen on a sterile operating table, make a spleen cell suspension with RPMI1640 medium, and mix it with conA (10ug / ml);
[0082] (2) After culturing for 3 days, add the above-mentioned transfection supernatant according to the concentrations diluted by 1 / 2, 1 / 4, 1 / 8, 1 / 20, and 1 / 50 of the stock solution, 50 μl / well, and set up triplicate wells for each concentration;
[0083] (3) Continue culturing for 24 hours, collect the cells, and measure the cytotoxicity (CD4 + : T cell membrane surface marker; IFN-γ: Th1 intracellular marker; IL-4: Th2 intracellular marker; CD19 + : B cell membrane surface marker);
[0084] (4) The results of flow cytometry showed that the number of T cells and Th1 cells decreased by 40%-60%, but there was no such effect on Th2 and B cells (such as figure 2 sho...
Embodiment 3
[0091] Example 3 Preliminary therapeutic effect of recombinant plasmids on autoimmune disease animal model EAE (experimental allergicencephalomyelitis)
[0092] 1. Establishment of EAE (Experimental Allergic Encephalomyelitis) animal model: C57BL / 6 mice were immunized with MBP according to conventional methods to establish an EAE model.
[0093] (1) Use C57BL / 6 mice to establish an EAE animal model, mix the self-extracted MBP crude extract with FCA (containing Mycobacterium tuberculosis 5mg / ml) in equal volumes, use a 3ml syringe to repeatedly push and pull to form a water-in-oil emulsion , by intraperitoneal injection.
[0094] (2) Immunization dose: each mouse was injected with 0.4ml of MBP: FCA (1:1) mixed solution, 0.2ml of pertussis bacilli liquid: PBS (1:50) mixed solution (containing 0.6-1.8×10 6 indivual).
[0095] (3) In the control group, each mouse was intraperitoneally injected with 0.2ml of a mixture of Bacillus pertussis liquid and PBS (containing 0.6-1.8×10 6...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com