Cannabinoid effervescent tablet and preparation method thereof
A technology of cannabinoids and effervescent tablets, which is applied in the direction of medical formulas, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., can solve the problem of low bioavailability, unstable CBD content, hemp seed oil, etc. Inconvenient to carry and store, etc., to achieve high bioavailability, short disintegration time, and quantifiable production indicators
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0127] Example 1: Preparation of water-soluble complex solution
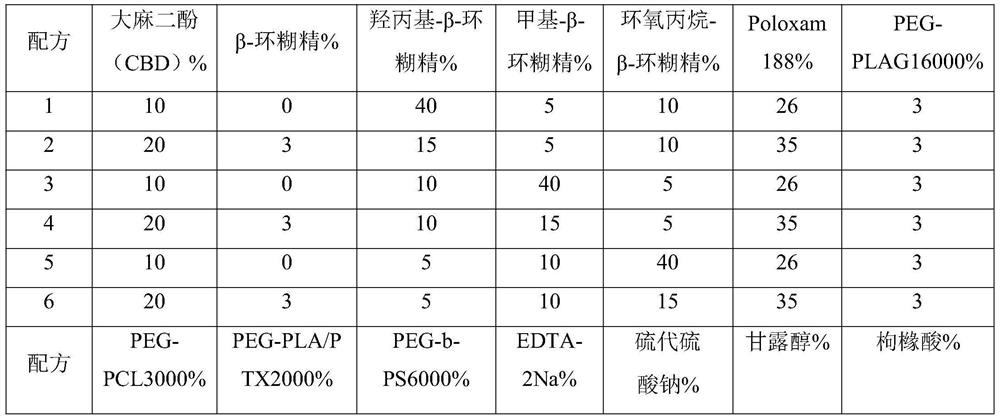
[0128] 1. The specific formula (weight percent content) of the water-soluble compound solution is shown in Table 1:
[0129] Table 1 Water-soluble compound solution formula table
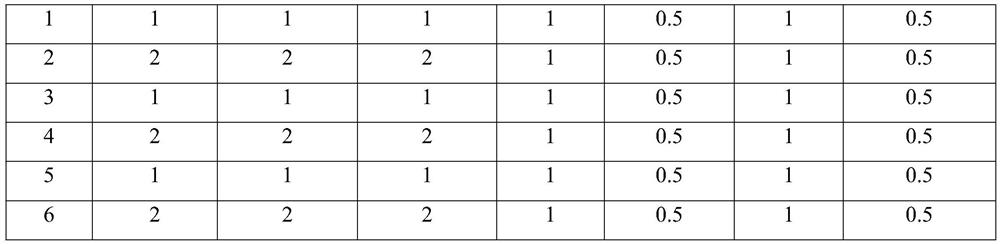
[0130]
[0131]
[0132] 2. Preparation process
[0133] (1) The first group of preparation processes (process 1)
[0134] Prepare the water-soluble compound solution according to the proportions of formulas 1-6, as follows:
[0135] a. Dissolution: β-cyclodextrin, hydroxypropyl-β-cyclodextrin, methyl-β-cyclodextrin, propylene oxide-β-cyclodextrin, Poloxamer 188, PEG-PLAG16000, PEG - One or more of PCL3000, PEG-PLA / PTX2000, PEG-b-PS6000, EDTA-2Na, sodium thiosulfate, mannitol and citric acid are added to 3 times of water and dissolved;
[0136] b. Mixing and dissolving: adding cannabidiol (CBD), stirring at 85° C. for 2 hours, the solution is a clear and transparent water-soluble compound solution after stirring, and the par...
Embodiment 2
[0149] Example 2: Preparation of Cannabinoid Composite Particles
[0150] The cannabinoid composite particles are obtained by drying the water-soluble composite solution obtained in Example 1, and the drying is freeze-drying or drying under reduced pressure.
[0151] 1. Freeze-drying process: put the water-soluble compound solution in Example 1 into a freeze-drying box, freeze at -40°C for 3h, vacuum at 100Pa, primary drying temperature 10°C, drying for 36h; secondary drying temperature 30°C, Dry for 24h; after drying, the material is sieved through No. 5 sieve to granulate;
[0152] 2. Decompression drying process: put the water-soluble compound solution in Example 1 into a decompression drying oven, the temperature is 50°C, the vacuum degree is 0.04Mpa, and it is dried for 48 hours. After drying, the material is passed through a No. 5 sieve to granulate.
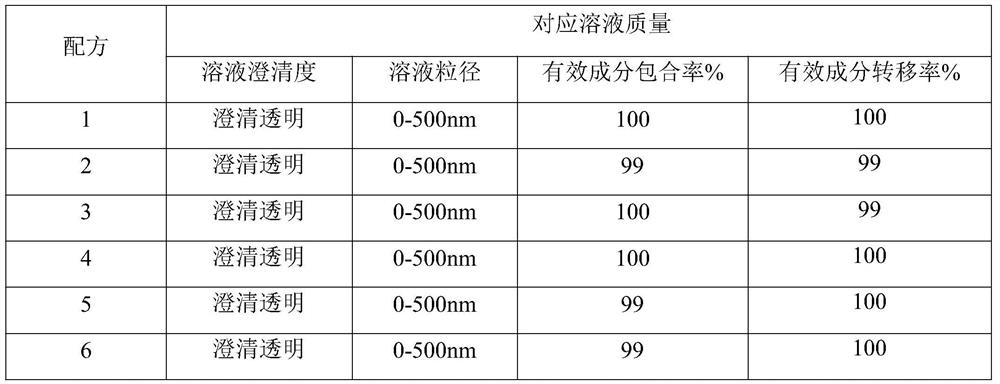
[0153] The comparison between freeze drying process and vacuum drying process is shown in Table 4:
[0154] Table 4 Co...
Embodiment 3
[0157] Example 3: Preparation of Cannabinoid Effervescent Tablets
[0158] 1. Formulation: cannabinoid-containing composite granules (powder obtained by drying process in Example 2), sodium bicarbonate, sodium carbonate, tartaric acid, citric acid, aspartame, direct-pressed lactose, sodium chloride, polyethylene glycol Alcohol, vitamin C, sucrose fine particles, apocarotene aldehyde, beta carotene, sucralose, essence, sodium fumarate, leucine. The specific formula (weight percentage content) is as follows:
[0159] Table 5 Formulation of cannabinoid effervescent tablets
[0160]
[0161] 2. Preparation process
[0162] According to the formula in Table 5, the cannabinoid composite particles (powder obtained by drying process in Example 2), sodium bicarbonate, sodium carbonate, tartaric acid, citric acid, aspartame, direct-pressed lactose, sodium chloride, polyethylene Diol, vitamin C, sucrose fine granules, apocarotene aldehyde, beta carotene, sucralose, essence, sodium ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle size (mesh) | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com