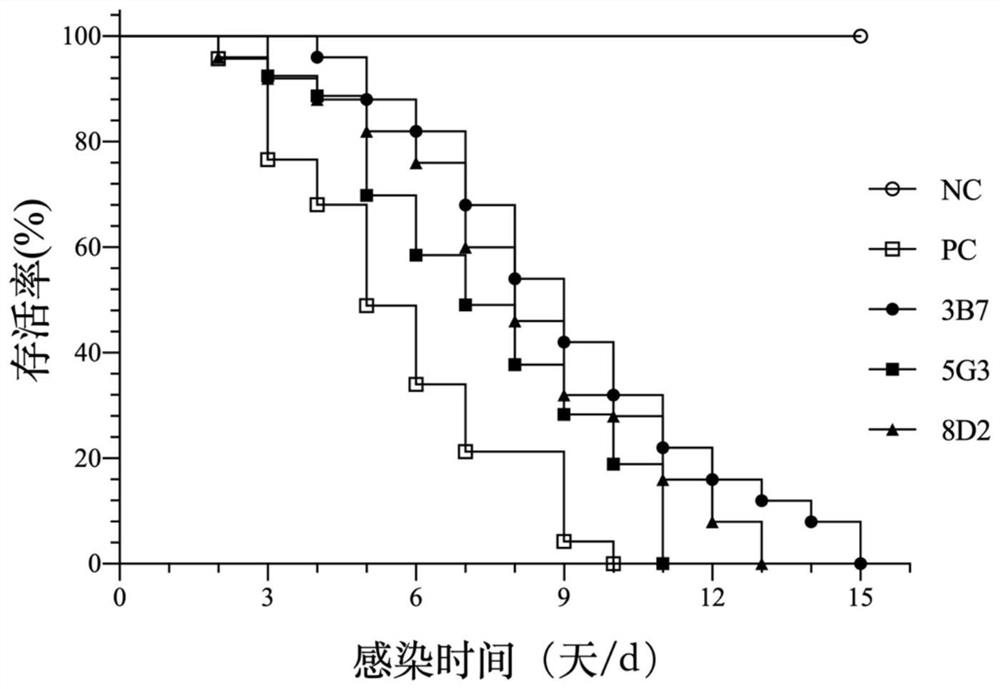
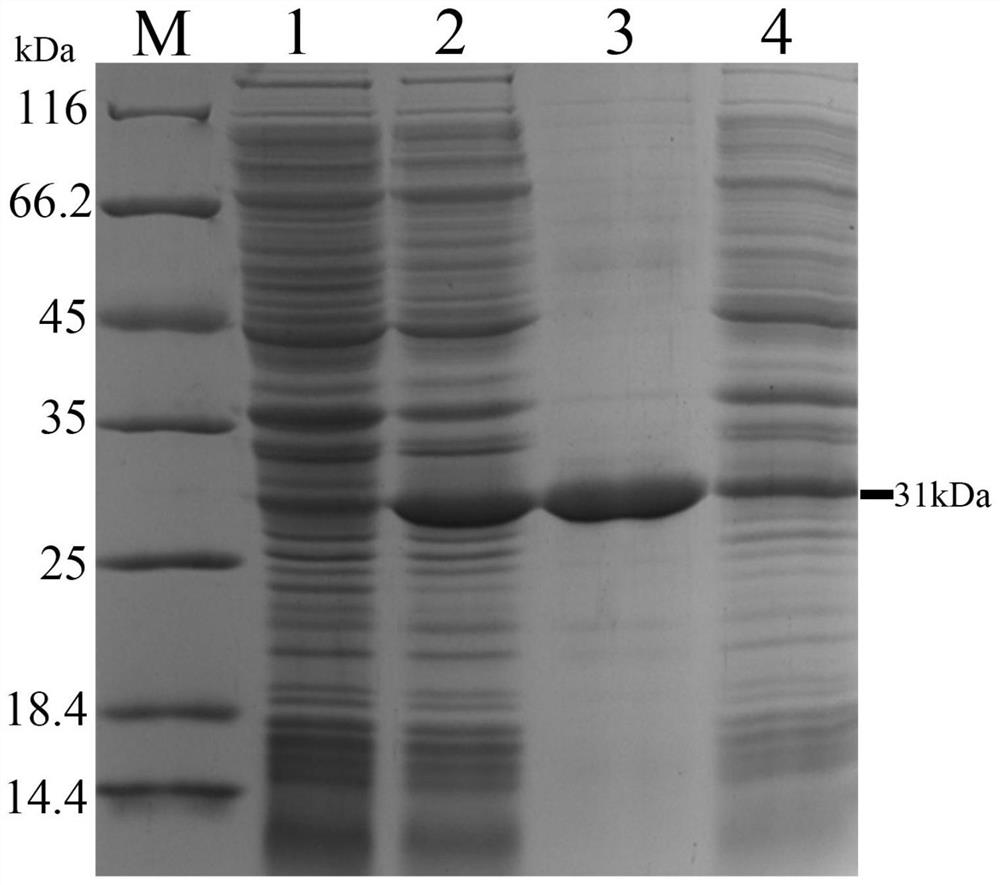
White spot disease virus single-chain variable region recombinant antibody as well as preparation method and application thereof
A recombinant antibody and variable region technology, applied in the fields of botanical equipment and methods, biochemical equipment and methods, antibodies, etc., can solve the problems of long time-consuming preparation of monoclonal antibodies, high cost of mass production, and poor tissue permeability, etc. Achieve fast and efficient immunogenicity, prevent infection and spread, and have strong tissue penetration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1: Preparation of recombinant protein of vitiligo virus envelope protein VP28 and development of neutralizing monoclonal antibody
[0037] 1. Preparation of VP28 recombinant protein of vitiligo virus envelope protein
[0038] (1) Based on the published WSSV genome sequence, a specific primer pair for VP28 was designed and used to amplify its open reading frame (ORF). All primers used for recombinant expression are listed in Table 1.
[0039] rVP28-F CGGGATCCATGGATCTTTCTTTCACTCTTTCGGTCG rVP28-R CCCAAGCTTCTCGGTCTCAGTGCCAGAGTAGGTG
[0040] (2) The amplified sequence was ligated into pET-32a vector and transferred into E. coli BL21 (DE3). Positive clones were screened by PCR and their correctness was confirmed by sequencing. Positive E. coli BL21 was grown in LB medium at 37°C to exponential growth phase, and then 100 mM IPTG was added to induce the expression of recombinant VP28.
[0041] (3) The His-tagged VP28 recombination was purified...
Embodiment 2
[0073] Example 2: Single-chain variable region antibody gene cloning of the VP28-neutralizing monoclonal antibody 3B7 of vitiligo virus
[0074] 1. Recovery of hybridoma cell lines, extraction of total RNA and synthesis of cDNA template
[0075](1) Take the cryopreserved anti-WSSV-VP28 neutralizing monoclonal antibody 3B7 hybridoma cell line out of the -80°C refrigerator, and immediately place it in a pre-heated 37°C water bath to thaw until it is completely thawed, and 1000 Centrifuge at room temperature for 3 minutes at rpm, carefully pour off the supernatant in the ultra-clean workbench, add 1ml of pre-warmed GIT cell culture medium at 37°C to gently suspend the cells, transfer to a 24-well cell culture plate, set with 4.5% CO 2 Cultured in a cell incubator.
[0076] (2) Take the cells in the logarithmic growth phase for total RNA extraction. The cells in the logarithmic growth phase are transparent, regular in shape, round and translucent. Collect about 1×l0 7 The hybri...
Embodiment 3
[0109] Example 3: Recombinant expression of single-chain variable region antibody of the present invention
[0110] (1) Use primer scFv-3B7V H F and scFv-3B7V L R to carry out the amplification of the scFv-3B7 gene, and the PCR product recovery kit to recover the target gene for use.
[0111] (2) Take the DNA fragment obtained in the previous step and the pET28a vector for double-enzyme digestion experiments.
[0112] (3) Configure the T4 DNAligase ligase system according to the molar ratio of the vector to the target gene of 1:10 (0.03pmol:0.3pmol), and react at 16°C overnight.
[0113] (4) All ligation systems were added to DH5α competent cells, and the transformation operation was carried out. The positive clones were screened by colony PCR and sent to the company for sequencing.
[0114] (5) Pick the correctly sequenced strains on the plate, culture them in liquid LB medium containing kanamycin overnight, extract the plasmids, and transform them into BL21 (DE3) competen...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com