Liquid crystal elastomer based on allyl selenide dynamic covalent bonds as well as preparation method and application of liquid crystal elastomer
A technology of allyl selenide and liquid crystal elastomer, applied in liquid crystal materials, chemical instruments and methods, organic chemistry, etc., can solve the problems of polarity and pH instability, material structure requirements, etc., and achieve good continuous performance , avoid irreversible damage, stimulate stable effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] Example 1: Preparation of Difunctional Hydroxyl Terminated Allyl Selenide Monomer
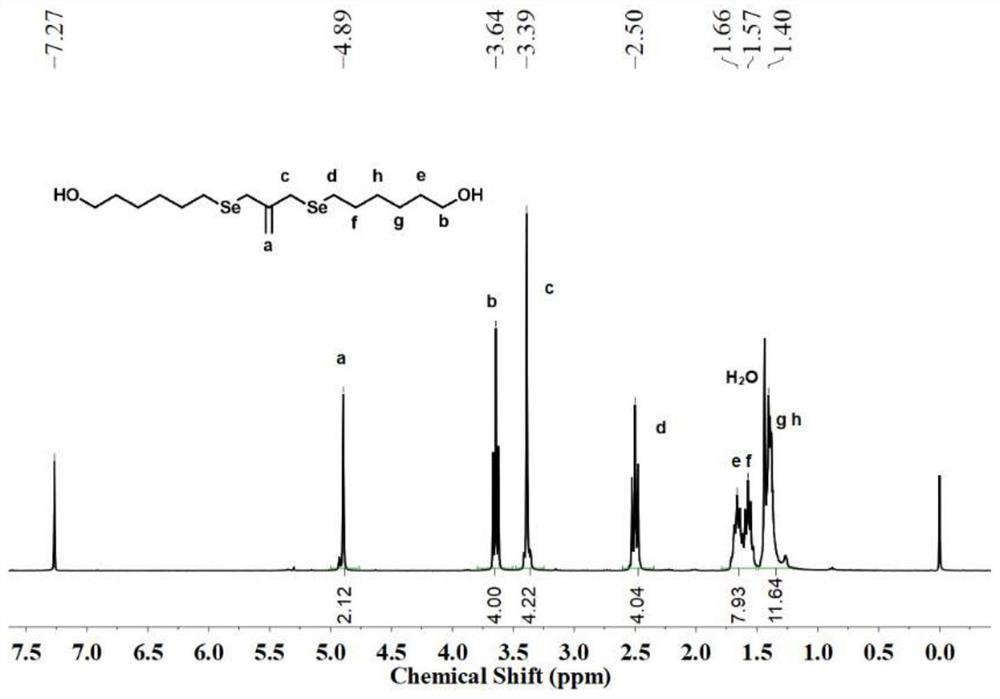
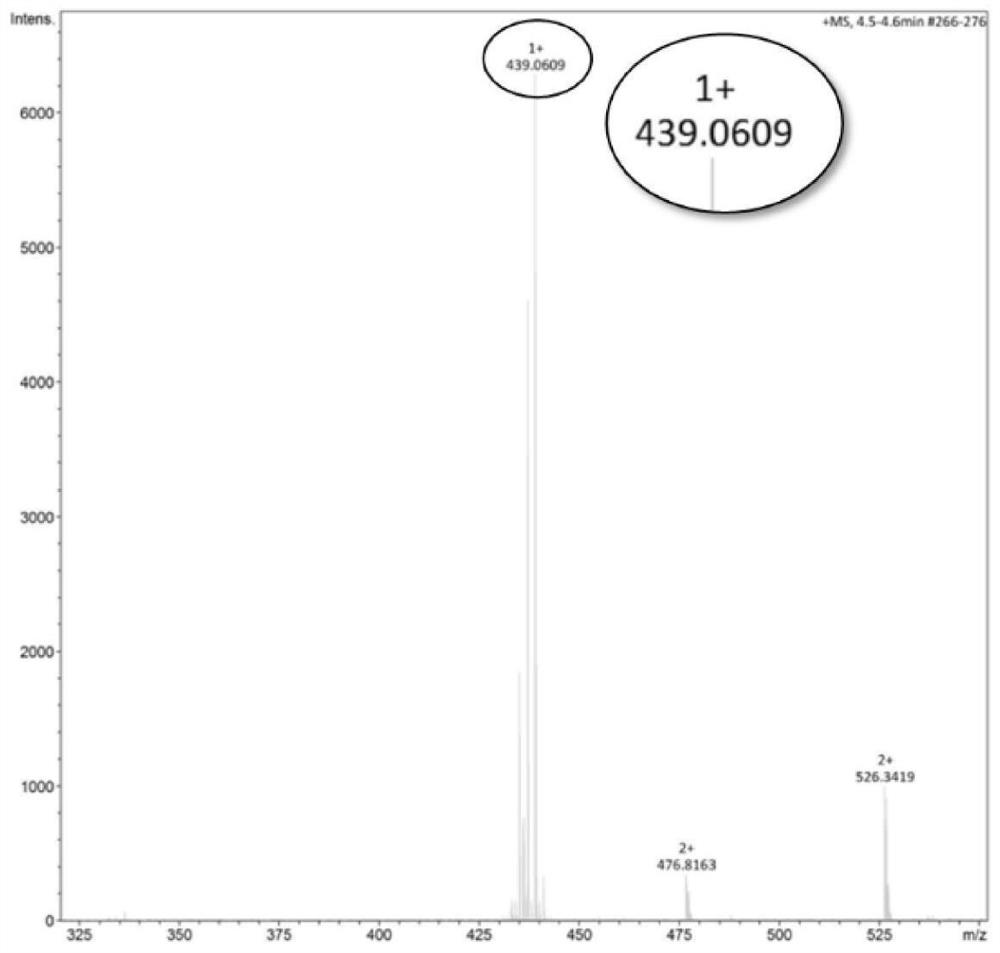
[0066] Selenium powder (800 mmol, 63.2 g) was weighed and placed in a 1 L three-necked flask, sodium hydroxide (750 mmol, 30.0 g) was dissolved in 400 mL of water, and then added to the three-necked flask, and argon was passed to remove oxygen. Sodium borohydride (107 mmol, 4.0 g) was dissolved in an aqueous solution of sodium hydroxide (2.0 g, 50 mL), slowly dropped into the above system, and reacted at room temperature for 2 h. 1 g of tetrabutylammonium bromide was added, and the temperature was raised to 50 °C. 6-Chloro-n-hexanol (400 mmol, 54.7 g) was dissolved in 400 mL of tetrahydrofuran, and the above system was added slowly. After reacting for 12 h, the reaction solution was extracted with ethyl acetate to obtain an organic phase, which was dried over anhydrous sodium sulfate, and separated by column chromatography to obtain hydroxyl-terminated diselenide (HO-C 6 -Se 2 ). wil...
Embodiment 2
[0070] Example 2: Preparation of Liquid Crystal Elastomer
[0071] Dissolve 1,4-bis[4-(6-acryloyloxyhexyloxy)benzoyloxy]-2-methylbenzene (2.5 mmol, 1.68 g) in 8 mL of tetrahydrofuran, dithiothreitol ( 2.0 mmol, 0.31 g) was dissolved in 2 mL of tetrahydrofuran, all were added to a 25 mL three-necked flask, after ventilation and deoxygenation, 50 μL of triethylamine was added. After 12h of reaction, the HO-C 6 -Se 1 (1 mmol, 0.41 g) and 1,6-hexamethylene diisocyanate (2 mmol, 0.34 g) were respectively dissolved in 2 mL of toluene and added to the three-necked flask in turn, and 100 ppm of dibutyltin dilaurate was added as a catalyst. After fully stirring, the mixture was dissolved in a 6×8 cm polytetrafluoroethylene mold, placed at room temperature for 4 hours to slowly volatilize the low-boiling organic solvent tetrahydrofuran, and then placed in a 60 ℃ oven for 12 hours to make the reaction fully, and then 100 Annealed at ℃ for 12h to obtain a liquid crystal elastomer.
[...
Embodiment 3
[0075] Example 3: Stretch orientation of liquid crystal elastomer
[0076] The obtained liquid crystal elastomer film was cut into a long strip, and a stable external force was applied to the sample strip at a rate of 10 mm / min at 50° C. to a strain of 50% with a stretcher. The sample was then rapidly cooled to room temperature, and the light intensity was 40 mWcm -2 Irradiated under 405 nm UV light for 1 h, the internal allyl selenide could undergo reversible addition-fragmentation chain transfer reaction, thereby obtaining a liquid crystal elastomer with successful orientation fixation.
[0077] Differential scanning calorimetry (DSC) curves and polarized light microscopy images proved that the liquid crystal elastomers were successfully oriented, and the results were as follows Figure 7-8 shown. It can be seen from the figure that the liquid crystal elastomer material has been successfully oriented, and its phase transition temperature (T NI) is 70°C. The corresponding...
PUM
| Property | Measurement | Unit |
|---|---|---|
| phase transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com