Catalpol nasal drops as well as preparation method and application thereof
A technology of nasal drops and catalpol, which is applied in the directions of blood diseases, pharmaceutical formulations, extracellular fluid diseases, etc., can solve the problem that patients cannot arrive at the hospital in time, and achieves reduction in the number of neuronal cell apoptosis in the cortex and stable storage properties. , good safety effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Embodiment 1, Zichun nasal drop prescription screening
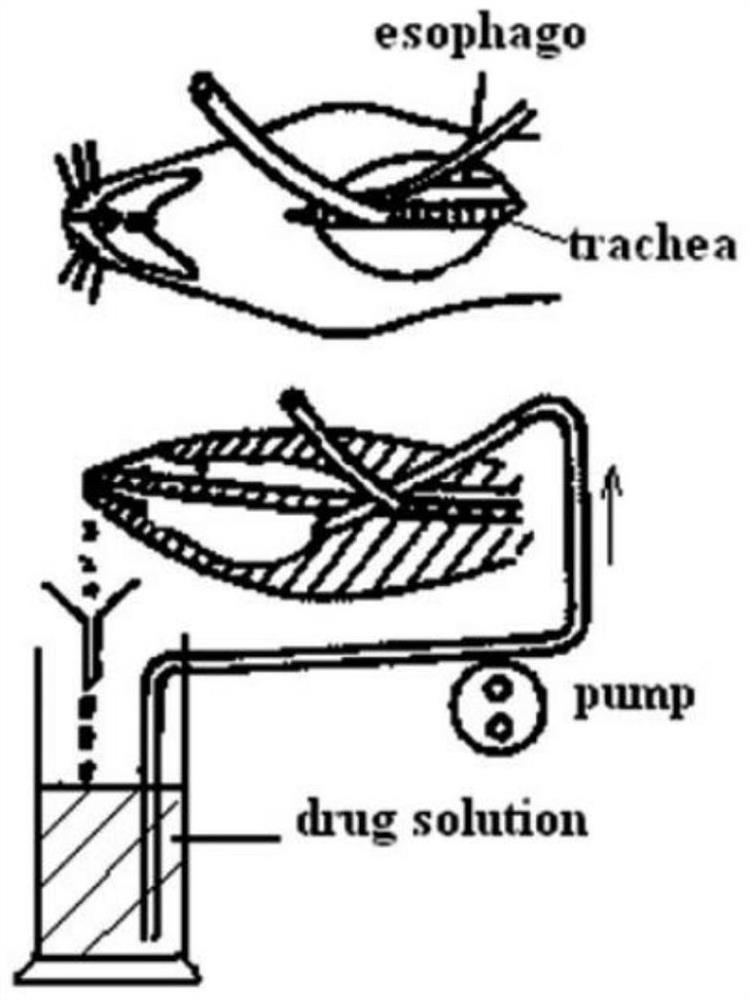
[0043] Due to the rich vascular system and the highly permeable structure in the nasal membrane in the nasal cavity, intranasal administration can achieve rapid onset of effect. However, there are two major obstacles to intranasal absorption of drugs, one is the low membrane permeability of polar drugs, and the other is that the drug is quickly cleared due to the mucociliary clearance, so adding safe and effective thickeners and absorption enhancers will can solve this problem to a large extent.
[0044]The pH value of nasal mucus can affect the action of solution bacterial enzymes and ciliary movement. The pH value of the normal nasal secretion fluid of adults is 5.5-6.5. When it is alkaline, it is easy to cause acute non-infectious inflammation. Exercise can cause bacterial infection. In addition, the previous experiments of the research group showed that catalpol is unstable to acid and alkali, so this part ...
Embodiment 2
[0084] Embodiment 2, the preparation technology and formula of Zichun nasal drops
[0085] According to the research of Example 1, the final concentration of each component of Zichun nasal drops is as follows, specifically shown in Table 6:
[0086] Table 6. Formula of catalpol nasal drops
[0087]
[0088] The specific preparation process is as follows: accurately weigh an appropriate amount of catalpol extract, prepare a catalpol solution with physiological saline, and prepare it as solution A, and reserve it for later use. Accurately weigh 0.1g of carbomer, add 50mL of normal saline to dissolve in a water bath at 40°C, and leave it at room temperature for several hours until the carbomer solution is observed to have no white core by naked eyes, and the swelling is complete, which is solution B. Add water-soluble azone to solution B and stir well, then add solution A and mix well again, adjust the pH value to 6.5-7.0 with phosphate buffer, set the volume to 100mL, and st...
Embodiment 3
[0090] Embodiment 3, the stability evaluation of catalpol nasal drops
[0091] Prepare catalpol nasal drop sample solution according to the prescription of embodiment 2, be divided into 4 parts and seal in test tube, respectively at normal temperature (4 ℃, 25 ± 2 ℃), high temperature 40 ℃, strong light irradiation (illuminance≧5000Lx fluorescent lamp) Preserve under the condition, in the 1st week, the 2nd week, the 3rd week, the 4th week get the solution and dilute to 1mg / mL respectively, measure the change of catalpol concentration according to the assay method of embodiment 1, observe the solution clarification situation and the change of pH value. Variety.
[0092] The experimental data was sampled with IBM spss20.0 (SPSS Inc, Chicago, USA) for one-way analysis of variance, and the statistical results were expressed as Mean ± SD. Use GraphpadPrsm5.0 (Graphpad Software, USA) to make statistics and make result graphs.
[0093] The stability test results of catalpol nasal dro...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com