HBV BCP region 1762/1764 mutation digital PCR detection kit and use method thereof
A detection kit, G1764A technology, applied in DNA/RNA fragments, microorganism-based methods, biochemical equipment and methods, etc., can solve the problems of cumbersome operation, false negative, poor discrimination of single nucleic acid mutation, etc., to improve accuracy , easy to operate, reproducible and specific results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
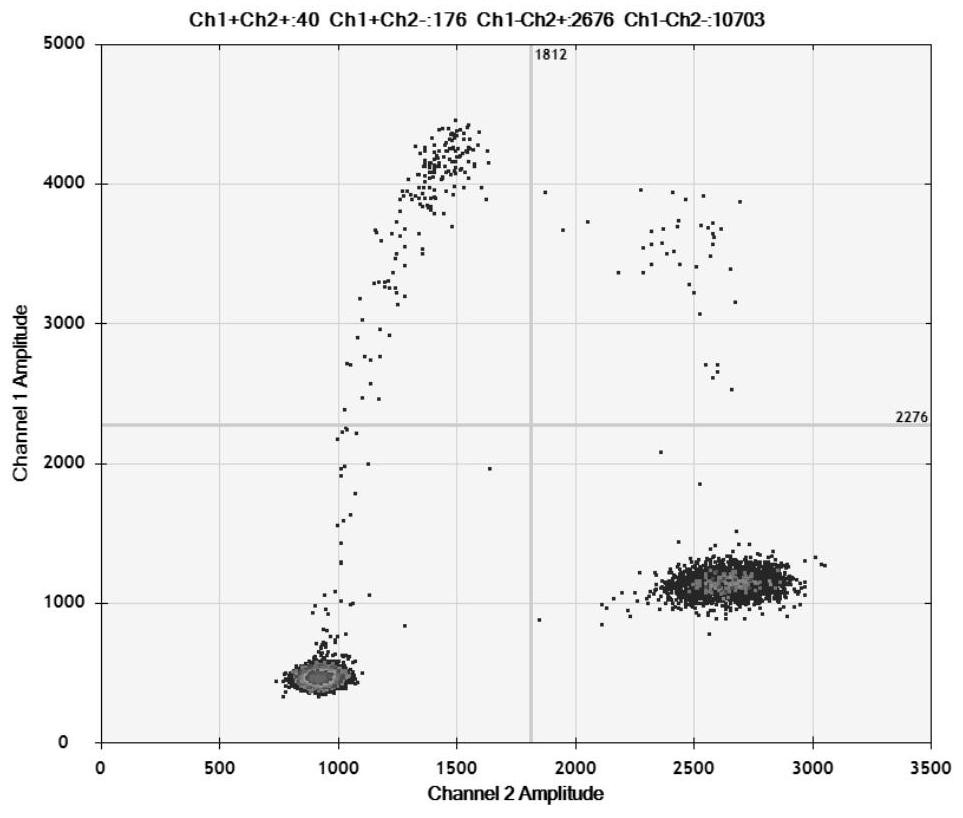
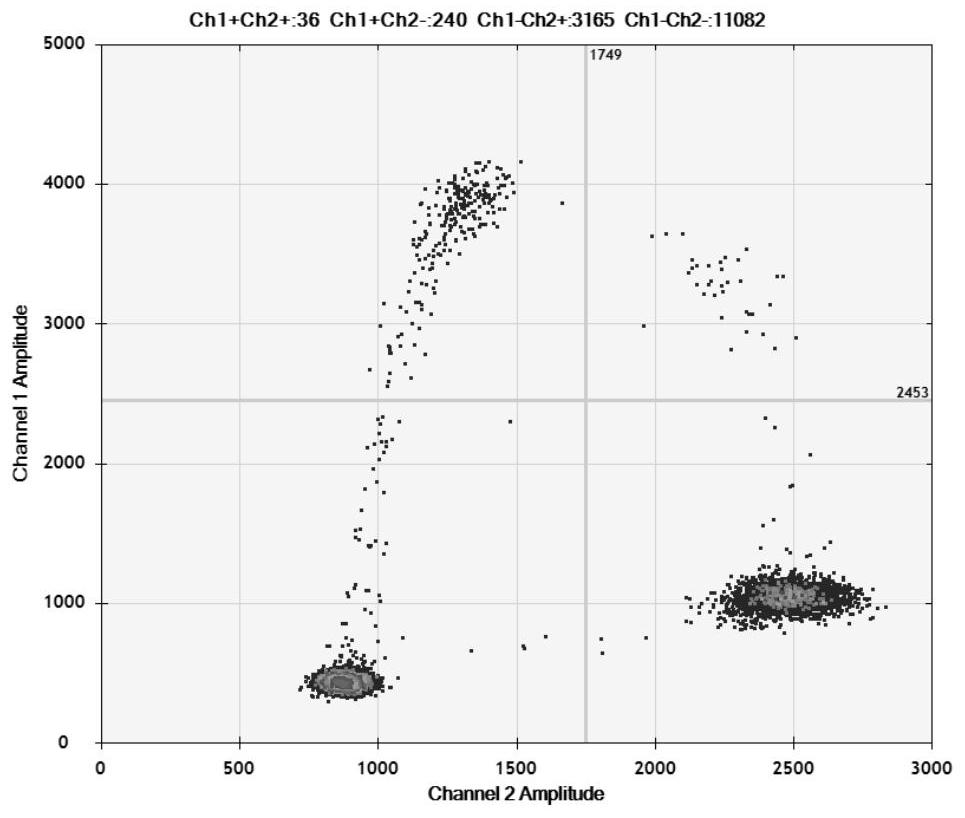
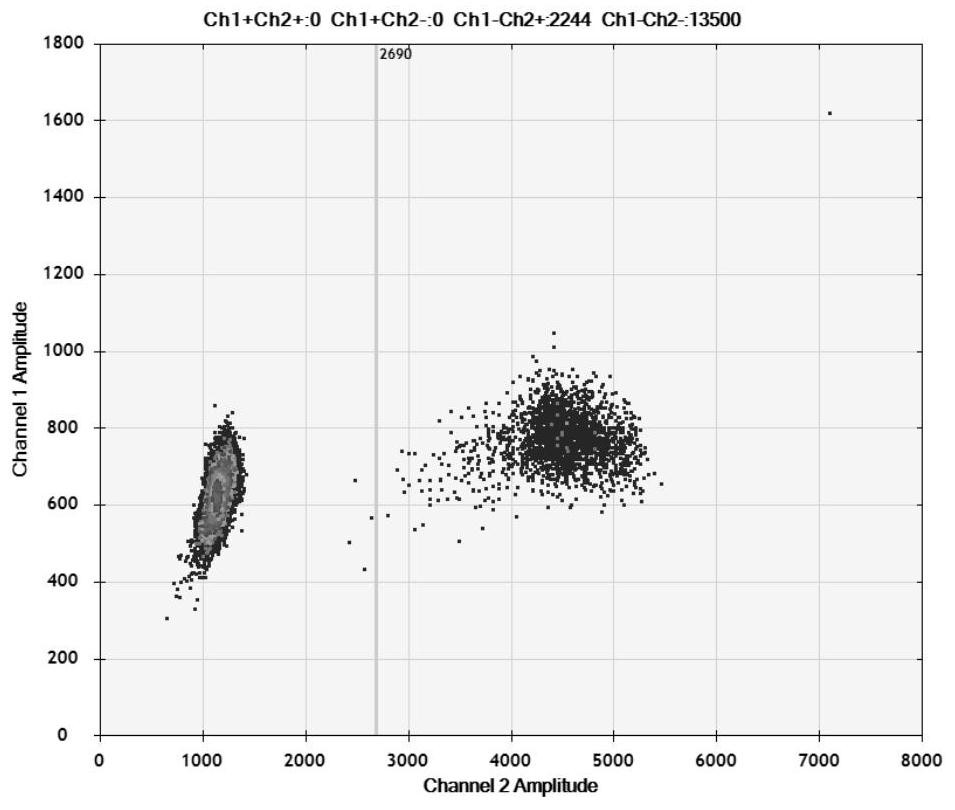
[0038] Example 1 HBV BCP region 1762 / 1764 mutation digital PCR detection kit
[0039] The HBV BCP region 1762 / 1764 mutation digital PCR detection kit of the present invention includes primers and probes for detecting two mutation sites of BCP region A1762T and G1764A.
[0040] The sequences of primers and probes for detecting the A1762T mutation site are shown in SEQ ID NO: 1-3; the sequences of primers and probes for detecting the G1764A mutation site are shown in SEQ ID NO: 4-6.
[0041] A1762T point mutation:
[0042] Upstream primer: 5'-CTGGGGGAGGAGATtAGGTt T A -3', as shown in SEQ ID NO:1;
[0043] Downstream primer: 5'-GAGATGACTAGGCAgAGGTgAAA-3', as shown in SEQ ID NO:2;
[0044] Probe: 5'-FAM-TTGCATGGTGCTGGTGAACAGACCAATT-BHQ1-3', as shown in SEQ ID NO:3;
[0045] Amplified product: CTGGGGGAGGAGATTAGGTT A A GGTCTTTGTACTAGGAGGCTGTAGGCATAAATTGGTCTGTTCACCAGCACCATGCAACTTTTTTCACCTCTGCCTAGTCATCTC, as shown in SEQ ID NO:13.
[0046] G1764A point mutation:
[0047] Upst...
Embodiment 2
[0069] Example 2 HBV BCP region 1762 / 1764 mutation digital PCR detection kit using method
[0070] The method of using the HBV BCP region 1762 / 1764 mutation digital PCR detection kit includes the following steps:
[0071] S1, 1762 / 1764 mutant HBV was diluted to 1000 copies / μL and 20 copies / μL with negative serum, wild-type HBV was diluted to 1000 copies / μL, the above three virus-containing sera plus negative serum were extracted with a commercial kit, and Perform digital PCR detection, 10 times for each detection, and evaluate the repeatability and accuracy of the detection method.
[0072] S2, prepare a single reaction system according to the components and concentrations of the PCR reaction solution in Table 1.
[0073] S3, sample addition: mix 15 μL reaction solution + 5 μL sample for each reaction tube and add sample.
[0074] S4, preparation of microdroplets: add 8 20 μL reaction systems to 8 wells in the middle row of DG8 cartridge. Add 70 μl of droplet generating oil...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com