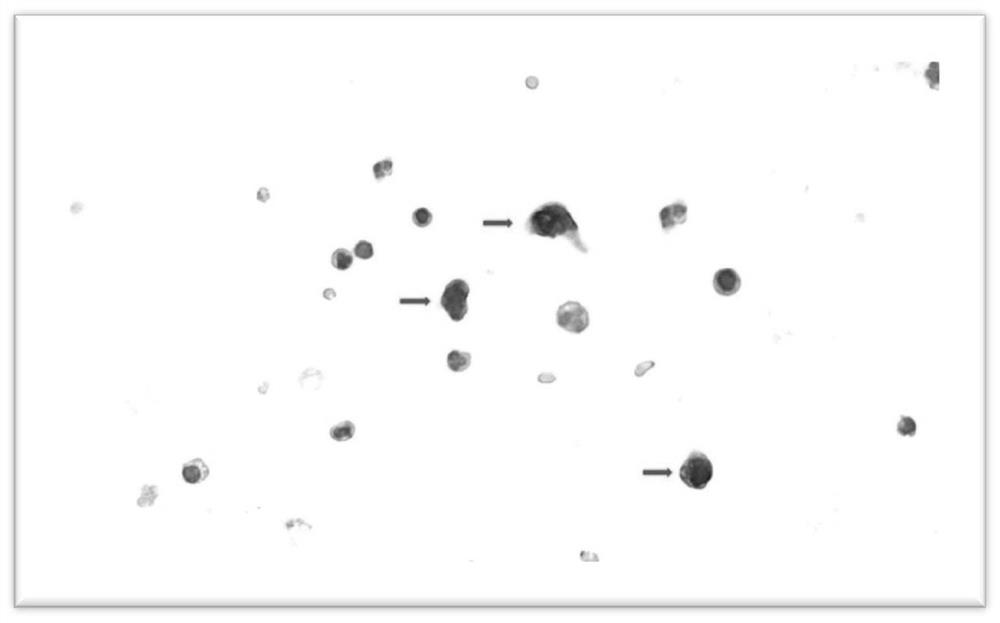
Manufacturing method of blood circulating tumor cell pathological chip
A tumor cell and chip technology, which is used in the detection of circulating tumor cells in the blood, can solve the problems of false positives, affecting the observation of morphological diagnosis, and unable to provide cell morphological characteristics.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0095] Blood sample preparation
[0096] 1. EDTA anticoagulant tube (purple) 10ml blood collection tube for taking patient's venous blood. A cell suspension containing 100 human urothelial carcinoma cell line T24 was added.
[0097] 2. Add 1 ml BD Lysing Solution 10X concentrate and incubate for 5 minutes to lyse red blood cells.
[0098] 3. Add 1 ml anti-human CD45 monoclonal antibody at a concentration of 0.5 μg / ml and incubate for 30 minutes.
[0099] 4. Add 1 ml of liquid guinea pig serum (G9774, Sigma-Aldrich) and incubate for 1 minute.
[0100] 5. Transfer the reaction liquid into a round bottom centrifuge tube, centrifuge at 1000rpm for 5 minutes, remove the supernatant, and obtain the cell pellet.
Embodiment 2
[0102] Liquid-based Immunochemical Staining Method for Circulating Tumor Cells
[0103] 1. The circulating tumor cell pellet obtained in Example 1 was diluted and suspended in 1 ml of PBS (pH7.4) buffer solution, and placed in a 10 ml plastic test tube.
[0104] 2. Add 20 microliters of Tween20 (to increase the permeability of the cell membrane, which is beneficial to the staining of the cell membrane and nucleus).
[0105] 3. Add 100 microliters of Dual Endogenous Enzyme Block (Dako, Carpinteria, Ca) to remove endogenous peroxidase (peroxidase) and alkaline phosphatase (alkline phosphatase) activity in cells, and incubate for 5 minutes.
[0106] 4. After centrifuging at 1000 rpm for 5 minutes, remove the supernatant and remove excess Dual Endogenous EnzymeBlock.
[0107] 5. Add 200 microliters of Serum-Free Block (Dako) and incubate for 10 minutes (to fill in non-specific protein binding sites).
[0108] 6. Add the primary antibody, ie, 200 microliters of mouse anti-human C...
Embodiment 3
[0116] Fabrication of Pathology Chip
[0117] The design of the chip is as follows:
[0118] 1. Pathological slides of regular size, 75 mm X 25 mm;
[0119] 2. The middle 40X20mm area (rectangle) contains cell adsorption material (100nm rough surface);
[0120] 3. 50X25 mm cover glass, 0.2 mm thick, containing microfluidic channels 1 mm wide and 50-100 microns high;
[0121] 4. The microfluidic channel is a serpentine single channel;
[0122] 5. The glass slide and the cover glass form a closed system, and the cell suspension passes through the microfluidic channel to ensure that the rare tumor cells are fully contacted with the adsorption material and are adsorbed on the glass slide;
[0123] 6. The valve parts of the liquid inlet and the liquid outlet are protruding, with a height of 0.5 cm, which is convenient for the equipment to pump the cell suspension;
[0124] 7. The position of the liquid inlet and the liquid outlet does not block the moving track of the conventio...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Height | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com