Nano-carrier for treatment of tumor through combination of chemotherapy and radiotherapy as well as preparation method and application of nano-carrier
A combination therapy and nanocarrier technology, applied in the field of biomedical nanomaterials, can solve the problems of short half-life, aggravate systemic side effects, etc., and achieve good therapeutic effect, good biosafety, and enhanced biocompatibility.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0037] For this reason, the present invention proposes a kind of preparation method that is used for the combination treatment nanocarrier of tumor chemotherapy and radiotherapy, comprises the following steps:
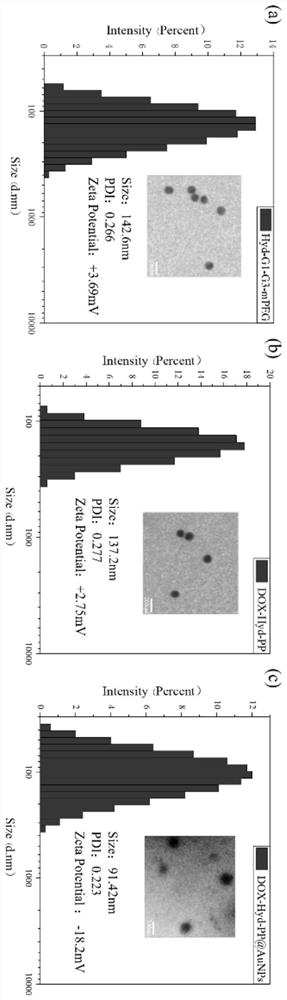
[0038] Step 1, select lysine as a raw material, use ethylenediamine as the core, and synthesize G1-G3 generation peptide dendrimers by a divergent method;
[0039] Step 2, modifying mPEG on the surface of G1-G3 peptide dendrimers as an acid-sensitive bond, and synthesizing Boc-Hyd-G1-G3-mPEG (Boc-Hyd-PP) nanoparticles as a carrier;
[0040] Step 3, linking the chemotherapeutic drug DOX with the obtained nanoparticles through a chemical bond to obtain DOX-Hyd-PP;
[0041] Step 4. Using the in-situ recombination method, the gold ions coated in the cavity of the nanoparticles are in-situ reduced, and the gold ions are reduced to nano-silver and gold loaded therein to obtain double drug-loaded nanoparticles DOX-Hyd-PP@AuNPs.
[0042] Wherein, the concrete processing of de...
Embodiment 1
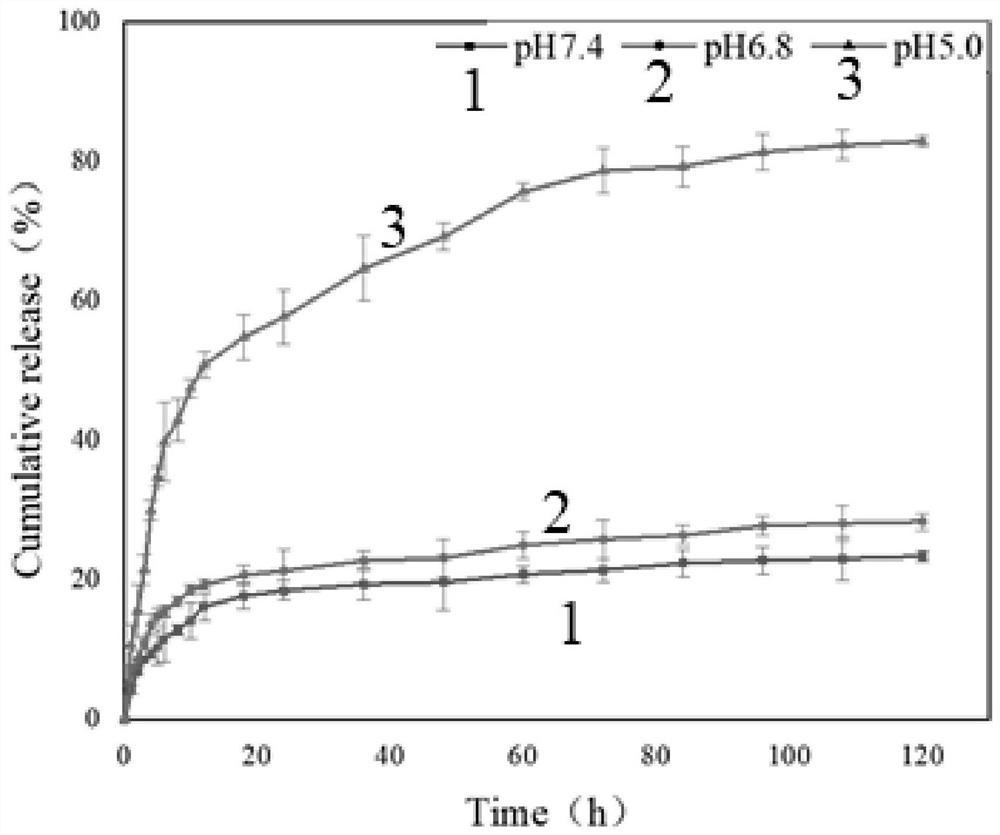
[0061] In order to determine the DOX release of drug-loaded materials in vitro, we simulated the in vivo conditions for drug release experiments. Weigh 4mg of DOX-Hyd-PP and dissolve it in 1mL of PBS buffer with different pH (pH5.4, 6.8, 7.4), transfer the liquid into a dialysis bag with a cut-off flow of 1000, clamp the dialysis bag and put it into 25mL of PBS buffer buffer (pH5.4, 6.8, 7.4) in a centrifuge tube. Put the centrifuge tube into a constant temperature shaker at 37°C and 120r / min to start timing. 1 mL was taken out at 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 12, 18, 24, 36, 48, 60, 72, 84, and 96 h and supplemented with PBS buffer of corresponding pH. The fluorescence absorption value of DOX at 480nm was measured with an ultraviolet spectrophotometer, and the amount of DOX released at different time points was calculated according to the DOX concentration-absorption intensity standard curve, and the cumulative release amount was calculated by taking the average value (n=3)...
Embodiment 2
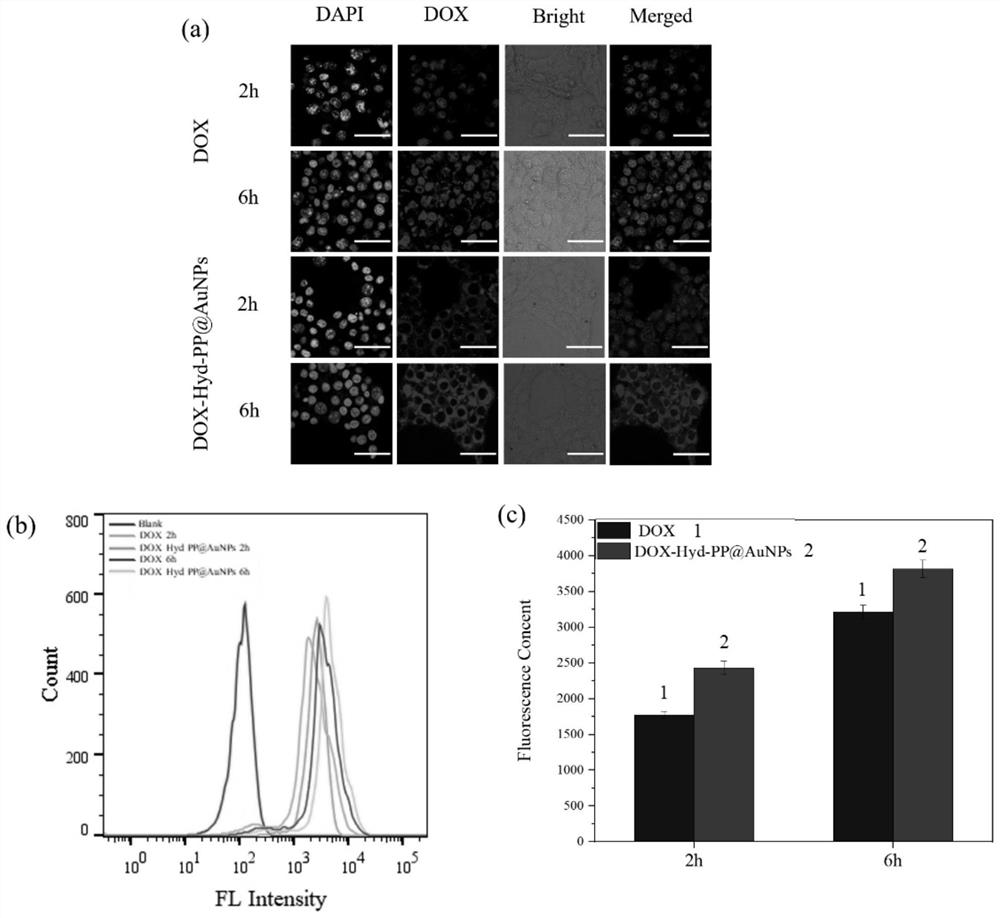
[0064] In order to track the phagocytosis of nanogels in cells, 4T1 cells were divided into 4 × 10 4 Cells were seeded at a confocal laser dish and incubated for 24 h. After the cells adhered to the wall, the medium was discarded, and fresh medium of DOX.HCl and DOX-Hyd-PP@AuNPs (DOX content of 5 μg / mL) was added to incubate for 2 h and 6 h, respectively. After the incubation, the medium was removed, washed three times with PBS, fixed with 4% paraformaldehyde for 15 min, and washed three times with PBS. Add ready-to-use DAPI staining agent for staining for 8 minutes, wash with PBS three times, add anti-fluorescence quenching mounting solution to seal the slide, and use laser confocal to observe the fluorescence intensity in the cells.
[0065] Then 4T1 cells were divided into 4 × 10 per well 5 Each cell density was seeded in a 6-well plate, and each group was set up with 3 replicate wells, and 2 mL of complete medium containing 10% fetal bovine serum was added to each well, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com