Responsive NO nano-drug as well as preparation method and application thereof
A nano-drug and drug technology, applied to nano-materials for the treatment of glaucoma, its preparation, in the field of responsive nitric oxide nano-drugs, can solve the problems of poor solubility and stability, low NO storage, limiting preclinical research and applications, and the like, To achieve the effect of convenient operation, simple preparation process and good application prospect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Example 1: Nanomedicine HOS-J R preparation of
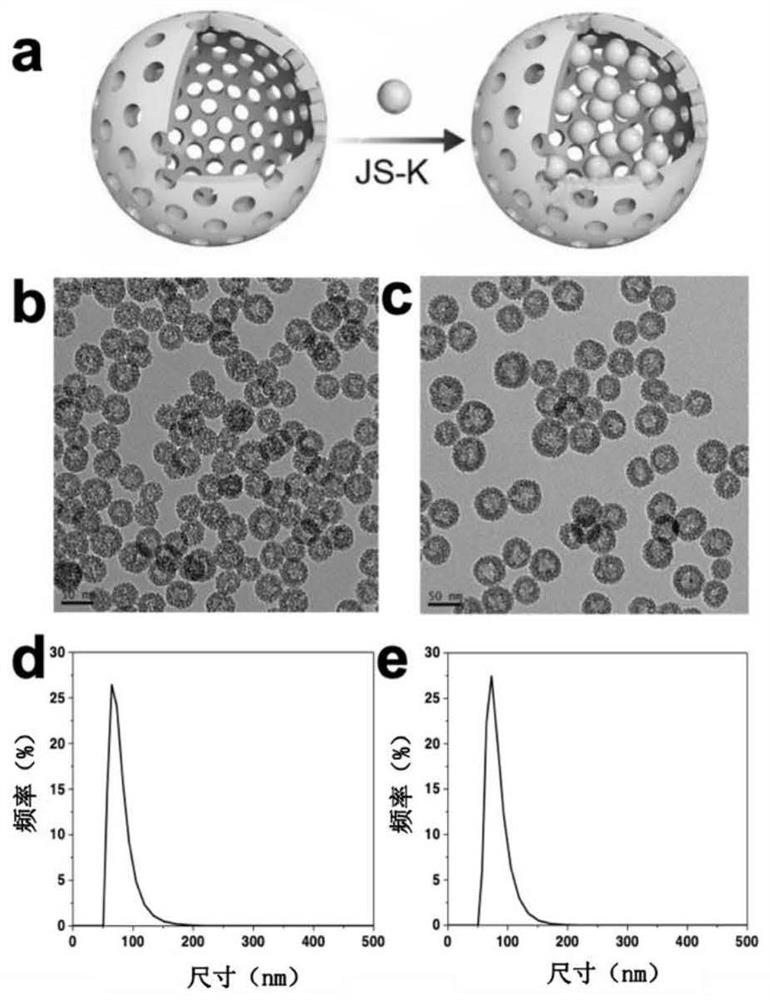
[0052] (a) Preparation of solid silica nanoparticles (MS): After stirring an aqueous solution of 2 g cetyltrimethylammonium chloride (CTAC) and 0.1 g triethanolamine (TEA) in a water bath at 95 °C, add 1 mL dropwise Tetraethyl orthosilicate (TEOS), react for 1h.
[0053] (b) Preparation of mesoporous silicone-coated solid nanoparticles (MS@MOS): adding bis-[r-(triethoxysilyl)propyl]-tetrasulfide (BTES) and TEOS to the above solution Mix the silicon source and react for 4h to obtain MS@MOS.
[0054] (c) Preparation of HOS: The above product was centrifuged, washed with ethanol, dispersed in a mixed solution of 100 mL ethanol and 10 mL concentrated hydrochloric acid (37%), and heated to 78° C. for 12 h to remove template CTAC. Repeat the above steps 3 times. After washing, redisperse in 20mL of water. Take 1 mL of the above solution and add 0.4 mL of ammonia water to react at 95°C for 3 h, selectively etch the MS core,...
Embodiment 2
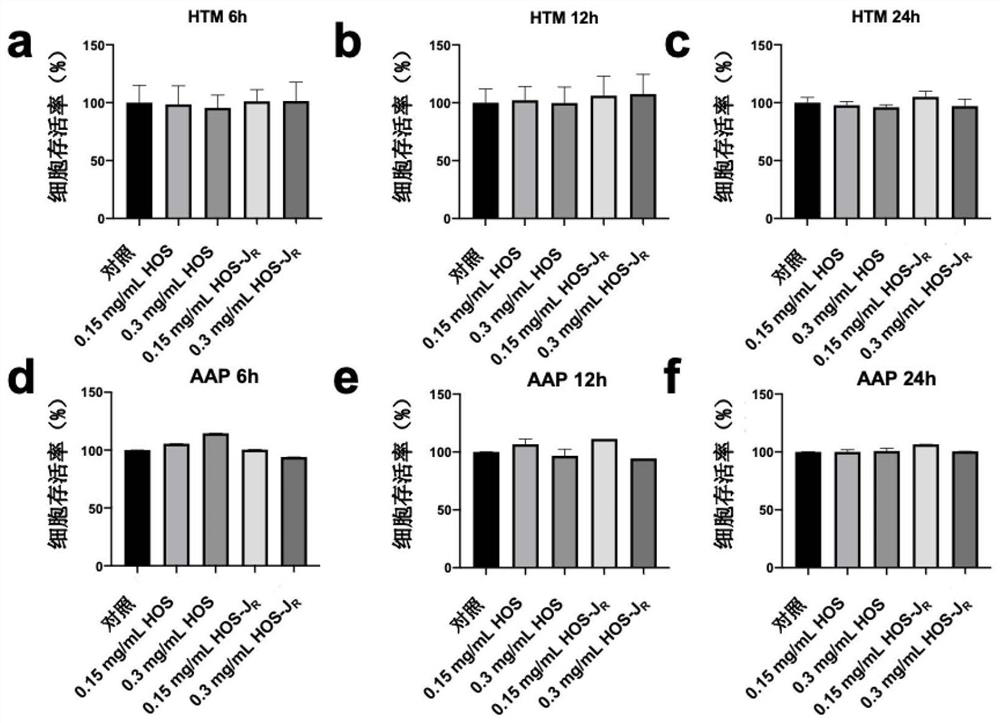
[0057] Example 2: Cytotoxicity Evaluation
[0058] HOS-J R Nanomedicine plays a role by reaching the target cells trabecular meshwork cells and Schlemm canal endothelial cells that regulate intraocular pressure, so the first evaluation of HOS-J R Effect on HTM cell viability. HTM cells were seeded into 96-well cell culture plates at a density of 5000 cells per well. After overnight incubation at 37° C., the cells were washed with PBS, and then 100 ul of fresh cell culture medium containing different concentrations (0.15 and 0.3 mg / mL) of drugs was added thereto and incubated for 6 h, 12 h and 24 h. After each time point was reached, 10 ul of CCK-8 solution (10%) was added to continue incubation for 1 hour. Use a microplate reader to measure the absorbance at 450nm. Set up a blank group (no inoculation of cells), a control group (cells plus a medium without drugs) and an experimental group (cells plus a medium containing drugs) in the experiment, and calculate the experiment...
Embodiment 3
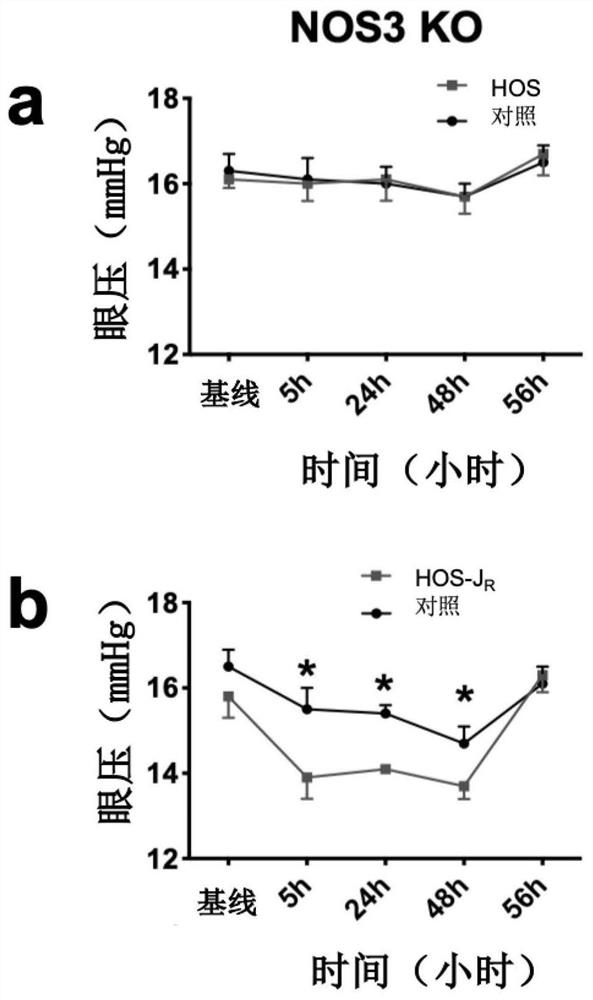
[0060] Embodiment 3: Pharmacological experiment
[0061] The intraocular pressure was measured at the same time period, and the mice were awake, using a Finnish animal-specific tonometer (model: tonolab) tonometer. When measuring the intraocular pressure, lightly grasp the skin of the neck behind the ear of the mouse with the left hand, make the mouse lie on the cage cover in a relaxed manner, and measure the intraocular pressure with the tonometer in the right hand. Measure three times and take the average value as an intraocular pressure measurement. Animal experiments were performed in accordance with the animal use and care system approved by the Animal Care and Use Committee of the Clinical Center of the National Institutes of Health. A mouse model of ocular hypertension - NOS3 knockout (NOS3 KO) mice was used in the experiments. NOS3 KO mice lack the NOS3 gene, resulting in loss of expression of the NO-producing endothelial nitric oxide synthase (eNOS) protein. Studie...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Hole size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com