A maf gene mutant, polypeptide, kit, construct, recombinant cell and application that cause congenital cataract
A congenital cataract and recombinant cell technology, applied in the direction of cells modified by introducing foreign genetic material, application, genetic engineering, etc., can solve the problem of hindering the clinical diagnosis of congenital cataract, rebirth, prenatal genetic diagnosis, new mutation data of MAF gene Imperfect and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
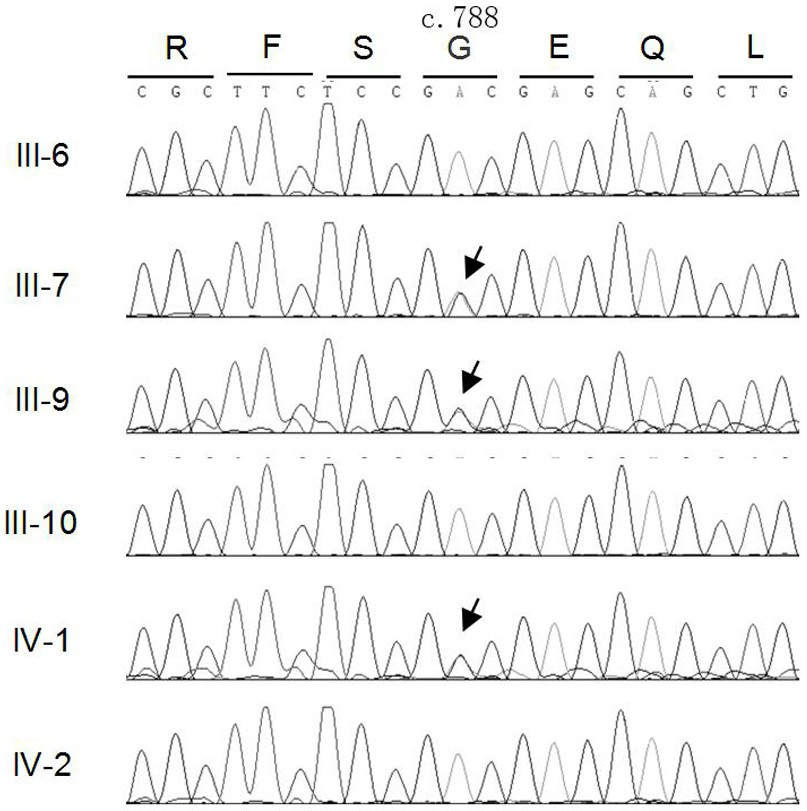
[0047] Design upstream and downstream amplification primers for the MAF gene mutation site, using the genomic DNA of cataract patients as a template, PCR method to amplify the upstream and downstream 341bp sequence of the MAF gene mutation site, the amplified product was subjected to agarose gel electrophoresis, and the PCR product After separation and purification, Sanger sequencing was performed to analyze the sequence and detect the mutation site of MAF gene in cataract patients.
[0048] Primers used to amplify the c.788A→G mutation site in exon 1 of MAF gene:
[0049] Forward 5'-GGCGGCCTGCACTTC-3' (SEQ ID NO.5);
[0050] Reverse 5'-GGGTTGTCGCTGCTCG-3' (SEQ ID NO. 6)
[0051] The reaction system for amplifying the sequence of the MAF gene mutation site by PCR method is shown in Table 1.
[0052] PCR reaction conditions: denaturation at 98°C for 3 minutes; then 11 cycles, denaturation at 98°C for 20 seconds, annealing temperature from 70°C to 60°C (1°C per cycle) for 20 s...
Embodiment 2
[0060] (1) Construction of MAF constructs with FLAG tag sequences (constructs of MAF wild type and c.788A→G mutants with FLAG tag sequences are shown in Figure 5 Shown): Design the upstream and downstream amplification primers for the full-length open reading frame of the MAF gene, the upstream primer has a Hind III restriction site, the downstream primer has a BamH I restriction site, and the MAF gene exists c.788A→G Genomic DNA of a cataract patient with heterozygous mutation is used as a template. Since one MAF allele of this patient is a wild-type normal sequence, and the other MAF allele contains a c.788A→G mutation sequence, using the patient’s genomic DNA as a template, PCR Methods Amplify the MAF open reading frame sequence, and simultaneously obtain the wild type and c.788A→G mutant MAF open reading frame sequences, and the amplified product is subjected to Hind III and BamH I double enzyme digestion and purification; commercialized pFLAG - After the CMV4 expression ...
Embodiment 3
[0100] The nucleic acid construct containing the wild-type MAF gene and the c.788A→G mutant constructed in Example 2 was used to transfect recipient cells to prepare recombinant cells.
[0101] Recipient cells were derived from Escherichia coli cells (DH5α competent cells, purchased from Tiangen Biochemical Technology Co., Ltd.) or mammalian cells (HEK293T cells, purchased from National Biomedical Experimental Cell Resource Bank).
[0102] The method for transforming DH5α competent cells with MAF constructs: 5 μL FLAG-tagged ligation products were transformed into 30 μL DH5α competent cells, placed on ice for 20 minutes, heat-shocked at 42°C for 1 minute and 30 seconds, incubated on ice for 5 minutes, and inoculated in ampicillin 30 μL of DH5α competent cells were transformed with 5 μL of GFP-labeled ligation products, placed on ice for 20 minutes, heat-shocked at 42°C for 1 minute and 30 seconds, incubated on ice for 5 minutes, and inoculated in kanamycin resistant agarose pl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com