Preparation method and equipment of clozapine dispersible tablet
A technology of clozapine and loose tablets, which is applied in the field of medicine, can solve the problems of uneven particle size, cumbersome production process, and low bioavailability, and achieve the effects of fast onset of action, high bioavailability, and uniform granulation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
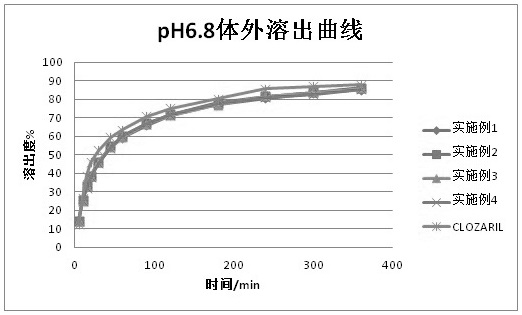
Examples
Embodiment 1
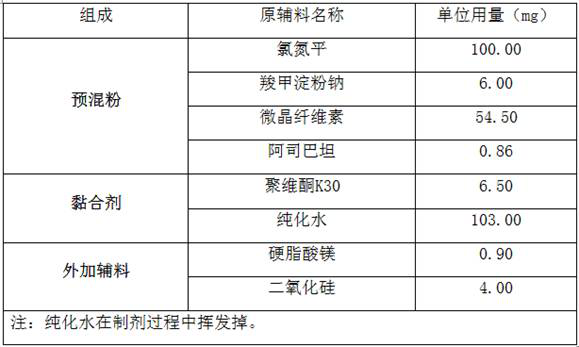
[0033] A kind of raw material of clozapine dispersible tablet is shown in the table below:
[0034]
[0035] A preparation method of clozapine dispersible tablet, specifically:
[0036] 1. Preparation of premixed powder: After passing clozapine, carboxymethyl starch sodium, microcrystalline cellulose and aspartame through a 40-mesh sieve, weigh them according to the prescription and mix them evenly to obtain a premixed material;
[0037] 2. Adhesive preparation: Weigh povidone K30 and purified water according to the prescription amount, dissolve povidone K30 in purified water until it is clear and transparent, and obtain the adhesive;
[0038] 3. Granulation: Put the premixed powder into the material chamber of the fluidized bed, turn on the air supply device and the turntable, adjust the turntable speed to 20rpm, set the air inlet temperature to 45°C, and the air inlet volume to 70m 3 / h, the atomization pressure of the spray gun is 0.2MPa, when the set temperature reache...
Embodiment 2
[0043]
[0044] A preparation method of clozapine dispersible tablet, specifically:
[0045] 1. Preparation of premixed powder: After passing clozapine, carboxymethyl starch sodium, microcrystalline cellulose and aspartame through a 40-mesh sieve, weigh them according to the prescription and mix them evenly to obtain a premixed material;
[0046] 2. Adhesive preparation: Weigh povidone K30 and purified water according to the prescription amount, dissolve povidone K30 in purified water until it is clear and transparent, and obtain the adhesive;
[0047] 3. Granulation: Put the premixed powder into the material chamber of the fluidized bed, turn on the air supply device and the turntable, adjust the turntable speed to 20rpm, set the air inlet temperature to 45°C, and the air inlet volume to 70m 3 / h, the atomization pressure of the spray gun is 0.2MPa, when the set temperature reaches 45°C, spray the binder into the material for granulation, the granules are gradually formed, a...
Embodiment 3
[0052]
[0053] A preparation method of clozapine dispersible tablet, specifically:
[0054] 1. Preparation of premixed powder: After passing clozapine, carboxymethyl starch sodium, microcrystalline cellulose and aspartame through a 40-mesh sieve, weigh them according to the prescription and mix them evenly to obtain a premixed material;
[0055] 2. Adhesive preparation: Weigh povidone K30 and purified water according to the prescription amount, dissolve povidone K30 in purified water until it is clear and transparent, and obtain the adhesive;
[0056] 3. Granulation: Put the premixed powder into the material chamber of the fluidized bed, turn on the air supply device and the turntable, adjust the turntable speed to 20rpm, set the air inlet temperature to 45°C, and the air inlet volume to 70m 3 / h, the atomization pressure of the spray gun is 0.2MPa, when the set temperature reaches 45°C, spray the binder into the material for granulation, the granules are gradually formed,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com