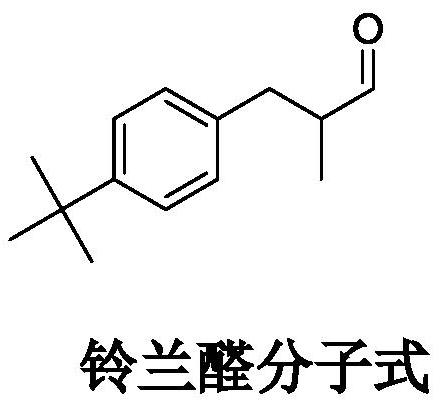
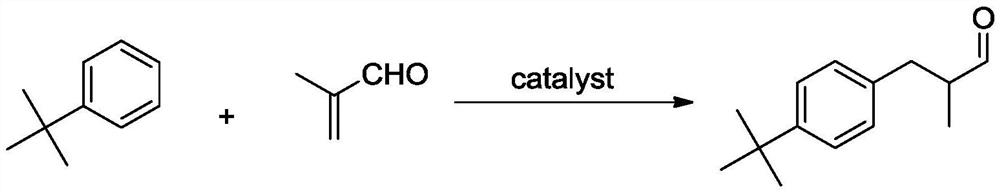
Preparation method of lilial
A technology of lilial and methacrolein, which is applied in the field of preparation of lilial, can solve the problems of high cost, high air tightness requirements, and high cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] 1) Preparation of Ti-K(Na) / F-Meso-SiO 2 catalyst:
[0044] To 200 g of deionized water was added 32 g (16 wt%) of mesoporous silica Meso-SiO 2 , drop concentrated ammonia water (26wt%) to adjust the system pH to 9.0, add 1.37g titanium tetrachloride (Meso-SiO 2 1.8wt% by mass as TiO 2 meter), heated to 21°C and stirred for 2.0h, then added 0.064g of NaF (Meso-SiO 2 0.2wt% of the mass), continue to stir for 3h, then place the solution in a rotary evaporator, adjust the temperature to 130°C, and the pressure is 30kPa (absolute pressure) to evaporate the solvent to dryness, then continue to rotary evaporator for 1.5h, and finally the solid Take it out and place it in a muffle furnace, and bake it at 500°C for 4 hours to get Ti-Na / F-Meso-SiO 2 catalyst.
[0045] 2) preparation of lyral
[0046] Add 64g α-methacrolein (32wt%) to 200g tert-butylbenzene, then add 6.0gTi-Na / F-Meso-SiO 2 (3.0wt%) catalyzer, and be placed in 0.5L reactor, temperature of reaction is raised ...
Embodiment 2
[0052] 1) Preparation of Ti-K / F-Meso-SiO 2 catalyst:
[0053] To 200 g of deionized water was added 34 g (17 wt%) of mesoporous silica Meso-SiO 2 , drop concentrated ammonia water (26wt%) to adjust the pH of the system to 8.5, add 2.52g titanium nitrate (Meso-SiO 2 2.0wt% by mass as TiO 2 meter), heated to 20°C and stirred for 2.5h, then added 0.102g of KF (Meso-SiO 2 0.3wt% of the mass), continue to stir for 4h, then place the solution in a rotary evaporator, adjust the temperature to 140°C, and the pressure is 35kPa (absolute pressure) to evaporate the solvent to dryness, then continue to evaporate and dry for 1.0h, and finally the solid Take it out and place it in a muffle furnace, and bake it at 450°C for 3 hours to get Ti-K / F-Meso-SiO 2 catalyst.
[0054] 2) preparation of lyral:
[0055] Add 68g α-methacrolein (34wt%) to 200g tert-butylbenzene, then add 8.0gTi-K / F-Meso-SiO 2 (4.0wt%) catalyzer, and be placed in 0.5L reactor, temperature of reaction is raised to 52...
Embodiment 3
[0057] 1) Preparation of Ti-Na / F-Meso-SiO 2 catalyst:
[0058] Add 20 g (10 wt%) of mesoporous silica Meso-SiO to 200 g of deionized water 2 , drop concentrated ammonia water (26wt%) to adjust the system pH to 8, add 2.37g titanium tetrachloride (Meso-SiO 2 5.0wt% by mass as TiO 2 meter), heated to 30°C and stirred for 0.5h, then added 0.12g of NaF (Meso-SiO 2 0.6wt% of the mass), continue to stir for 2h, then place the solution in a rotary evaporator, adjust the temperature to 150°C, and the pressure is 20kPa (absolute pressure) to evaporate the solvent to dryness, then continue to rotary evaporator for 0.5h, and finally the solid Take it out and place it in a muffle furnace, and bake it at 400°C for 6h to get Ti-Na / F-Meso-SiO 2 catalyst.
[0059] 2) preparation of lyral:
[0060] Add 50g α-methacrolein (25wt%) to 200g tert-butylbenzene, then add 4.0gTi-Na / F-Meso-SiO 2 (2.0wt%) catalyzer, and be placed in 0.5L reactor, temperature of reaction is raised to 80 ℃, reactio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com