A near-infrared fluorescent probe for detecting cytochrome p450 2C9 and its application
A cytochrome and fluorescent probe technology, applied in the field of near-infrared fluorescent probes, can solve the problems of drug side effects and increased drug exposure level, and achieve the effect of sensitive detection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
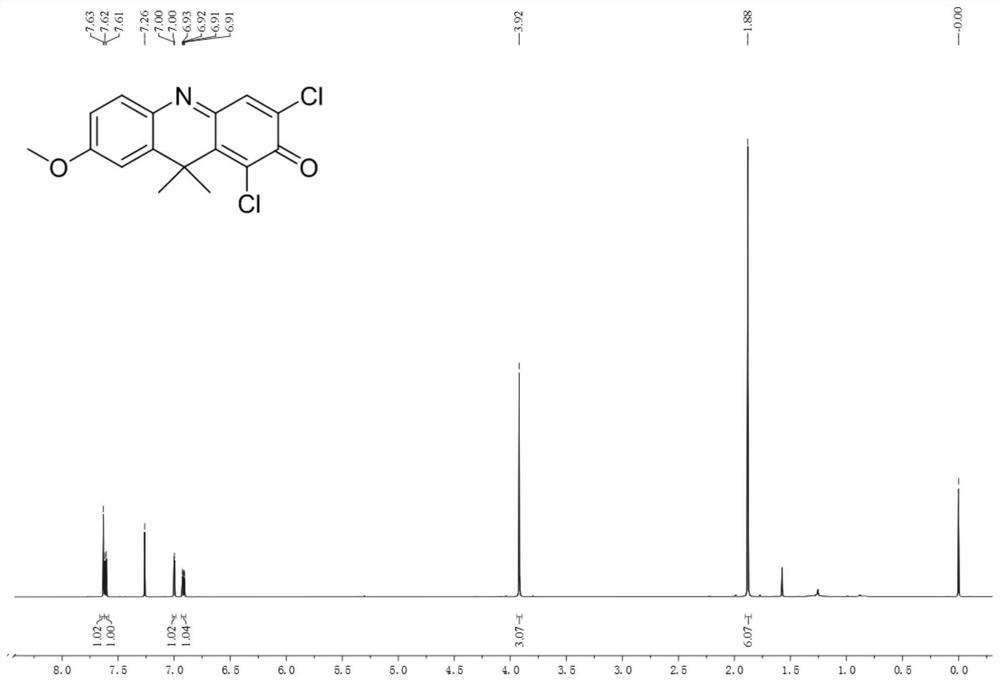
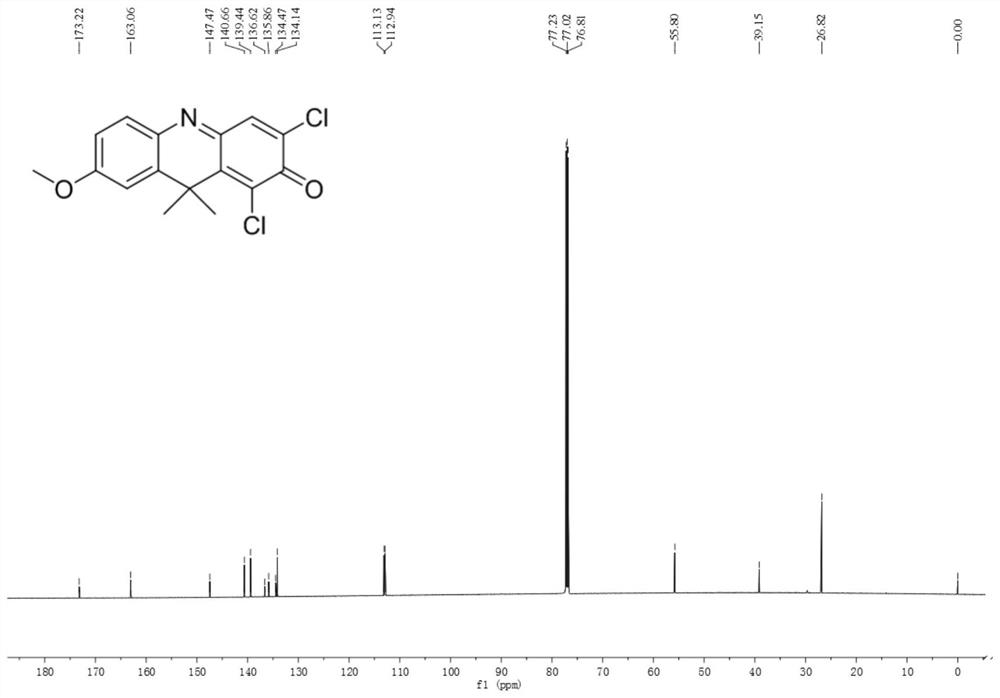
[0034] Example 1. 1,3-Dichloro-7-methoxy-9,9-dimethyl-2(9H)-acridone
[0035] Dissolve 61.6 mg (0.2 mmol) of 1,3-dichloro-7-hydroxy-9,9-dimethyl-2(9H)-acridone in 30 mL of acetonitrile, add 69.5 mg (0.5 mmol) of carbonic acid Potassium and 31 μL (0.5 mmol) methyl iodide were heated to reflux for 8 hours, cooled to room temperature, filtered, and acetonitrile was removed by distillation under reduced pressure. The remaining solid was dissolved in dichloromethane and separated by silica gel thin layer chromatography. The developing solvent was dichloro Methane: n-hexane=1:1 (volume ratio), 15.6 mg of orange solid was obtained as 1,3-dichloro-7-methoxy-9,9-dimethyl-2(9H)-acridone. Characterized by H NMR, C NMR and high-resolution mass spectrometry, the data are as follows: 1 HNMR (600MHz, CDCl 3 )δ7.63(s,1H),7.61(d,J=8.7Hz,1H),7.00(d,J=2.7Hz,1H),6.92(dd,J=8.7,2.7Hz,1H),3.92( s,3H),1.88(s,6H). 13 C NMR (150MHz, CDCl 3)δ173.22,163.06,147.47,140.66,139.44,136.62,135.86,134.47,1...
Embodiment 3
[0037] Example 3. Human recombinant CYP single enzyme incubation experiment
[0038] (1) Prepare CYP metabolism reaction incubation solution in advance, including pH 7.4 phosphate buffer (100mM), human recombinant CYP single enzyme, 1,3-dichloro-7-methoxy-9,9-dimethyl-2 The concentration of (9H)-acridone was 10 μM, and it was pre-incubated with shaking at 37°C for 3 minutes;
[0039] (2) Add 20 μL of 10 mM NADP to the reaction system + Solution initial reaction;
[0040] (3) After 30 minutes of reaction, 100 μL of ice acetonitrile was added, and the reaction was terminated after vigorous shaking;
[0041] (4) The samples were centrifuged at high speed for 20 minutes at 4°C and 20,000×g, and the supernatant was taken for fluorescence detection (E x =600nm, E m = 658 nm).
Embodiment 4
[0042] Example 4. Quantitative assessment of CYP2C9 activity in individual human liver microsomes from different sources
[0043] (1) A number of individual human liver microsomes (HLM) were selected and added to the reaction system for in vitro incubation experiments. Incubation system includes pH 7.4 phosphate buffer (100 mM), human liver microsomes, glucose 6-phosphate (10 mM), glucose-6-phosphate dehydrogenase (1 unit / mL), MgCl 2 (4mM), 1,3-dichloro-7-methoxy-9,9-dimethyl-2(9H)-acridone (10μM), pre-incubated with shaking at 37°C for 3 minutes;
[0044] (2) Add 20 μL of 10 mM NADP to the reaction system + initial reaction;
[0045] (3) After 30 minutes, 100 μL of ice acetonitrile was added, and the reaction was terminated after vigorous shaking;
[0046] (4) Under the conditions of 4°C, 20,000×g, centrifuge at high speed for 20 minutes, take the supernatant, and perform fluorescence detection (E x =600nm, E m =658nm), the rate of HLM-catalyzed dealkylation of 1,3-dichl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com