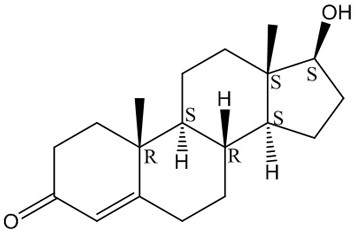
Carbonyl reductase mutant and application thereof in preparation of steroid hormone-testosterone
A mutant, reductase technology, applied in the field of bioengineering, to achieve the effects of low cost, high activity and mild catalytic reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
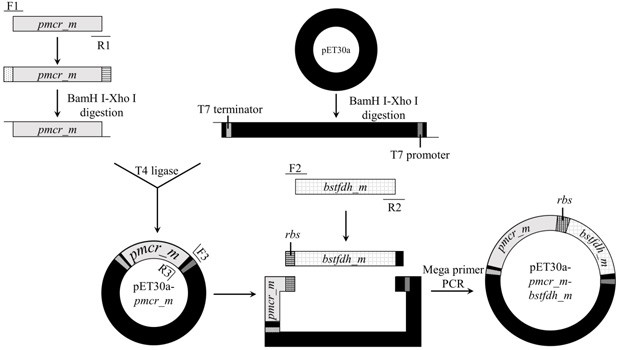
[0024] Example 1 Construction of engineering strains co-expressing carbonyl reductase mutant (PmCRm) and formate dehydrogenase (BstFDHm)
[0025] (1) PCR amplification of carbonyl reductase mutant gene pmcr_m and purify
[0026] The whole build process is as follows figure 2 shown.
[0027] The carbonyl reductase mutant PmCRm is obtained by mutating the 136th leucine into serine in the amino acid sequence shown in SEQ ID No.1 (WP_060395036.1).
[0028] According to the amino acid sequence (SEQ ID No.2) of the carbonyl reductase mutant PmCRm, with ( E. coli ) BL21 (DE3) was obtained by codon optimization for the target host pmcr_m Gene (SEQ ID No.3), and entrusted Shanghai Jierui Bioengineering Co., Ltd. to synthesize it. Two primers were designed according to the sequence shown in SEQ ID No.3:
[0029] F1 (SEQ ID No. 4):
[0030] 5'-ATC GGATCC ATGTCAGCATCTAAAAC-3' (the underline is the BamH I restriction site);
[0031] R1 (SEQ ID No. 5):
[0032] 5'-CAG CTCGA...
Embodiment 2
[0070] Example 2 Preparation of co-expression engineering strain quiescent cells
[0071] to pick (E. coli) BL21(DE3)-pET30a- pmcr_m-bstfdh_m A single colony of the engineering strain was added to 20 mL of LB liquid medium supplemented with 50 mg / L kanamycin, and cultured at 37°C and 200 rpm for 8-12 h as the seed solution. According to the inoculation amount of 2vol%, transfer to a 1 L shake flask containing 300 mL of LB medium supplemented with 50 mg / L kanamycin, and continue to culture until OD 600 0.6-0.7, then add lactose at a final concentration of 8% (m / v), place at 25°C and continue to shake for 12 h to induce the expression of the target gene. Then the above culture was centrifuged at 7000 rpm at 16°C for 3 min, the supernatant was discarded, the wet cells were collected, weighed and stored at -20°C for future use.
Embodiment 3
[0072] Example 3 The method of co-expressing engineering bacteria resting cells to convert androst-4-ene-3,17-dione into testosterone
[0073] According to the method of Example 2, resting cells of co-expression engineering strains were prepared and used as biocatalysts.
[0074] Determination of the product by high performance liquid chromatography: Elite high performance liquid chromatography, chromatographic column: Chiralpak AD-H Column (4.6 mm × 250 mm, 5 µm), mobile phase: n-hexane-isopropanol (v / v = 90 / 10), detection wavelength: 254 nm, column temperature: 30°C, flow rate: 1.4 mL / min. Under the detection conditions, the peak eluting times of androst-4-ene-3,17-dione and testosterone were 10.39 min and 11.47 min, respectively.
[0075] (1) Take the wet bacteria and suspend them in 100 mL phosphate buffer (100 mM, pH=7.5) to make the wet bacteria concentration 200 g / L, add the substrate androst-4-ene-3,17-dione 2.88 g, Tween 80 4 mL, co-substrate sodium formate 1.02 g,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Specific rotation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com