A kind of perch rhabdovirus recombinant g2 protein and its application
A rhabdovirus and protein technology, applied in the field of perch rhabdovirus recombinant G2 protein, can solve problems such as difficulty in meeting large-scale industrialization requirements, restriction, enzymatic inactivation, etc., and achieve good immunogenicity and immune protection performance. , good protective effect, good protective effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
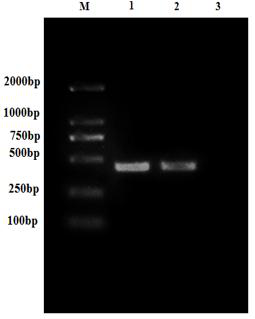
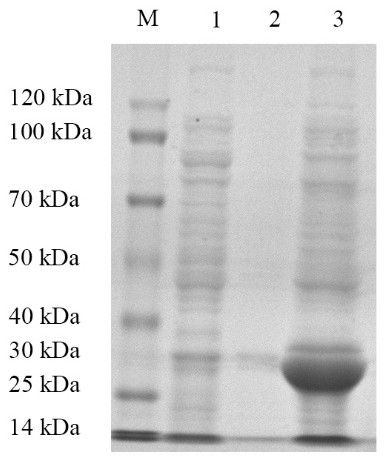
[0033] Embodiment 1: Recombinant Escherichia coli E. coli Construction and identification of BL21 / pET32a-G2 strain
[0034] G2 target gene amplification
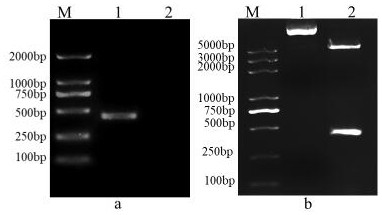
[0035] Referring to the instructions of the viral RNA extraction kit, the MSRV FJ985 strain virus liquid cultured with CO cells (the MSRVFJ985 strain was provided by Northwest A&F University, see Fei Yang et al. Evaluation on the antiviral activity of ribavirin against Micropterus salmoides rhabdovirus (MSRV) in in vitro and in vivo , Aquaculture, Volume 543 , Internet release time: May 27, 2021) as materials to extract MSRV virus RNA. Agarose gel electrophoresis was used to detect the integrity of the RNA, and a micro-nucleic acid analyzer was used to determine the concentration and quality of the sample RNA. The extracted RNA samples were reversed into cDNA using a reverse transcription kit.
[0036] After a large number of designs, the G2 fragment with the amino acid sequence of SEQ ID No: 2 was selected, and ...
Embodiment 2
[0053] Example 2: Evaluation of immune efficacy of recombinant protein
[0054] During the research and development process, different fragments were designed, and different glycoprotein fragments and complete glycoproteins were prepared using the method of Example 1, glycoprotein fragment G1 (amino acid sequence is SEQ ID No: 3), glycoprotein fragment G3 (The amino acid sequence is SEQ ID No: 4) protein and the complete glycoprotein (the amino acid sequence is SEQ ID No: 5), respectively verifying the immune efficacy of the above protein fragments. Specifically, the following immune efficacy evaluation test was adopted: 300 healthy perch were randomly divided into 6 groups, including the control group (PBS), the G1 protein group (40 mg / L), the G2 protein group (40 mg / L), and the G3 protein group. (40 mg / L), G protein group (40 mg / L), G protein group (80 mg / L), all sea bass were vaccinated with the corresponding vaccines through the bath solution and soaked for 6 hours. Subse...
Embodiment 3
[0056] Embodiment 3: the preparation of subunit vaccine
[0057] Functional modification of single-walled carbon nanotubes
[0058] The structure modification of single-walled carbon nanotubes is carried out by the mixed acid oxidation method, and the steps are as follows:
[0059] (1) Put 2g single-walled carbon nanotube sample into 150mL concentrated H 2 SO 4 and 50mL concentrated HNO 3 placed in a magnetic stirrer at 100 rpm for 48 h at room temperature;
[0060] (2) Filter the mixed acid mixture of carbon nanotubes obtained in the previous step with a circulating water vacuum pump, wash with pure water until the pH of the liquid is 7.4, then dry it at 60°C, and grind it into powder in a mortar , through a 300-mesh sieve, and the resulting product is functionalized single-walled carbon nanotubes (o-SWCNTs).
[0061] Preparation of carbon nanotube carrier subunit vaccine
[0062] Take the acidified single-walled carbon nanotube sample (0.5g) and add it to 2-(N-morpholi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com