Application of monoketene monocarbonyl curcumin analogue in preparation of antioxidant drugs
An anti-oxidation and drug technology, applied in the direction of drug combination, ketone active ingredients, anti-toxic agents, etc., can solve the problems of no pharmacological activity and high toxicity, and achieve the effects of reduced toxicity, improved stability, and good application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] The synthesis of embodiment 1 compound
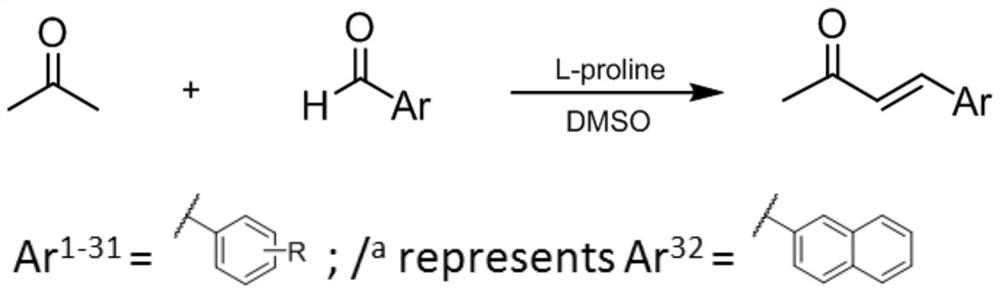
[0034] Using L-proline as a catalyst, monoketene monocarbonyl curcumin analogs were synthesized in one step. All compounds were obtained by reacting acetone with corresponding aldehydes at room temperature. After purification by column chromatography, as shown in Table 1, relatively high yields (40%-71%) were obtained.
[0035]
[0036] Synthesis and structure of monoketone monocarbonyl curcumin analogues. Reaction Conditioning: (I) L-Proline, DMSO, room temperature.
[0037] Table 1. Structures and yields of monoketene monocarbonyl curcumin analogues
[0038]
[0039] The structure of the compound was identified by mass spectrometry and H1-NMR, and the spectroscopic and physical and chemical data are as follows
[0040] . 1. (E)-4-phenylbut-3-en-2-one (sAc or sAc1)
[0041] Yellow powder, 70% yield, mp 34.8~36.0℃. 1 H-NMR (600MHz, CDCl 3 ),δ: 7.558-7.542(m,2H,Ar-H 2 ,β-H),7.520(d,J=16.2Hz,1H,Ar-H 6 ),7.409-7.398(m...
Embodiment 2
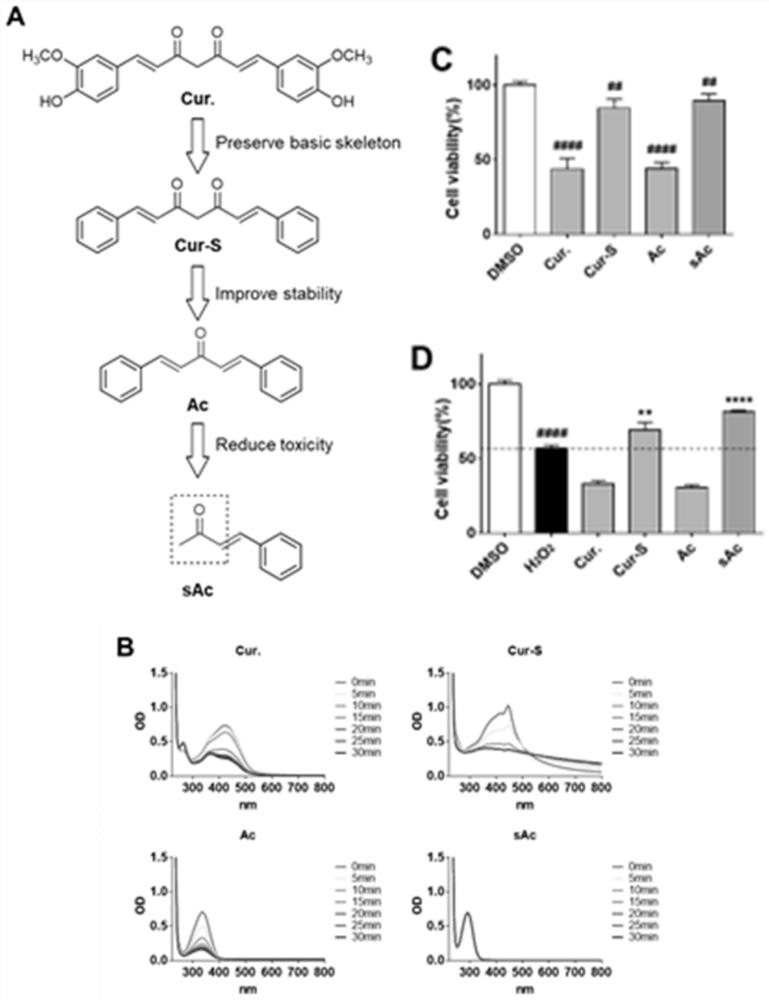
[0106] The monocarbonyl curcumin analog molecular skeleton (sAc or sAc1) has good stability, low toxicity, and good activity on cell protection.
[0107] In phosphate buffer, measured curcumin (Cur.), curcumin skeleton (Cur-S), diketene monocarbonyl curcumin analog molecule skeleton (Ac) and monoketene monocarbonyl curcumin of the present invention The stability of the molecular skeleton (sAc) of the analogue ( figure 1 B). The results showed that within 30 min, the light absorption of Cur., Cur-S, Ac decreased greatly in a time-dependent manner, while sAc remained unchanged. Therefore, it is suggested that sAc has a more stable structure than Cur., Cur-S, and Ac.
[0108] The effect of diketene monocarbonyl curcumin analog molecular framework (Ac) and monoketene monocarbonyl curcumin analog molecular framework (sAc) of the present invention on normal liver cells (MIHA) and kidney cells was determined by MTT method (NRK) cytotoxicity ( figure 2 ). The results showed that...
Embodiment 3
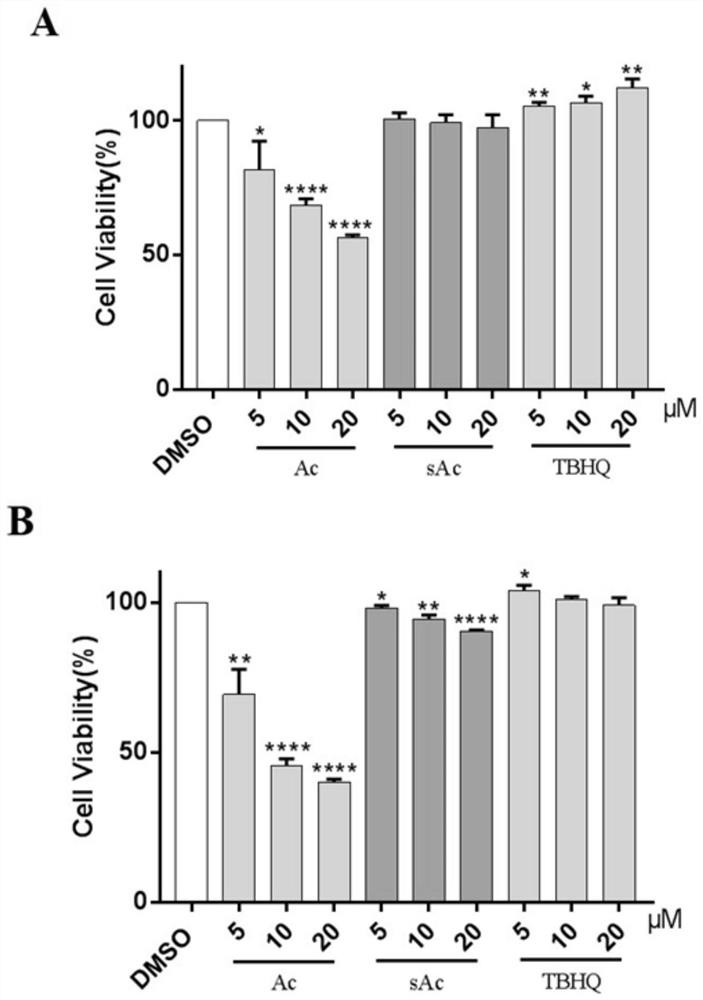
[0112] Monocarbonyl curcumin analogues (sAcs) have good antioxidant protective activity and low cytotoxicity against PC12 cells
[0113] The cytotoxicity of 32 sAc compounds to PC12 cells was measured by MTT method, and the results showed that, compared with the curcumin control group, all compounds clearly showed significant cytotoxicity to PC12 cells at the measured concentration ( Figure 4 A).
[0114] In H2O2-induced PC12 cell oxidative damage model, the antioxidant protection activity of 32 sAc compounds was determined. The results showed that cell viability increased to 70%–80% in most groups pre-incubated with compounds ( Figure 4 B). The protective activity of 20 compounds (sAc(1-5), sAc7, sAc9, sAc14, sAc(15-24), sAc27 and sAc32) was even better than that of the antioxidant TBHQ. The cell survival rate of the curcumin group was lower than that of the H2O2 group.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com