Recombinant L-arabinose isomerase LPAI and construction method and application thereof
A technology of arabinose and construction method, applied in the field of bioengineering, can solve problems such as high cost and low conversion efficiency, and achieve the effect of solving low conversion efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Embodiment 1: Recombinant E. coli BL21 / pANY1- araA build
[0035] (1) Lactobacillus bruceioids published in the Genbank database ( Lactobacillus parabuchneri ) of the coded L-AI araA The gene sequence (NCBI accession number: NZ_CP029256.1) was used as a template, combined with the characteristics of the multi-cloning site and homology arm on the vector pANY1 (from Shenyang Agricultural University), and the upstream primer F was designed and synthesized by using the bioinformatics Snapgene software:
[0036] 5'- GGGGGATCCACTAGTAGGCCT ATGTTACAAGTACCTGATTA-3' and downstream primer R: 5'- CA GGAGCTCCCATGGAGGCCT CTACTTGATGTCGTCAATG-3’ (the underlined parts are Stu I restriction site, the wavy line is the upstream and downstream homology arms homologous to the pANY1 plasmid).
[0037] (2) Using the genomic DNA of Lactobacillus brucelli as a template, use CWBIO Pfx high-fidelity polymerase (from Jiangsu Kangwei Century Biotechnology Co., Ltd.) to PCR amplify the ...
Embodiment 2
[0040] Example 2: Induced expression and purification of recombinant L-arabinose isomerase LPAI
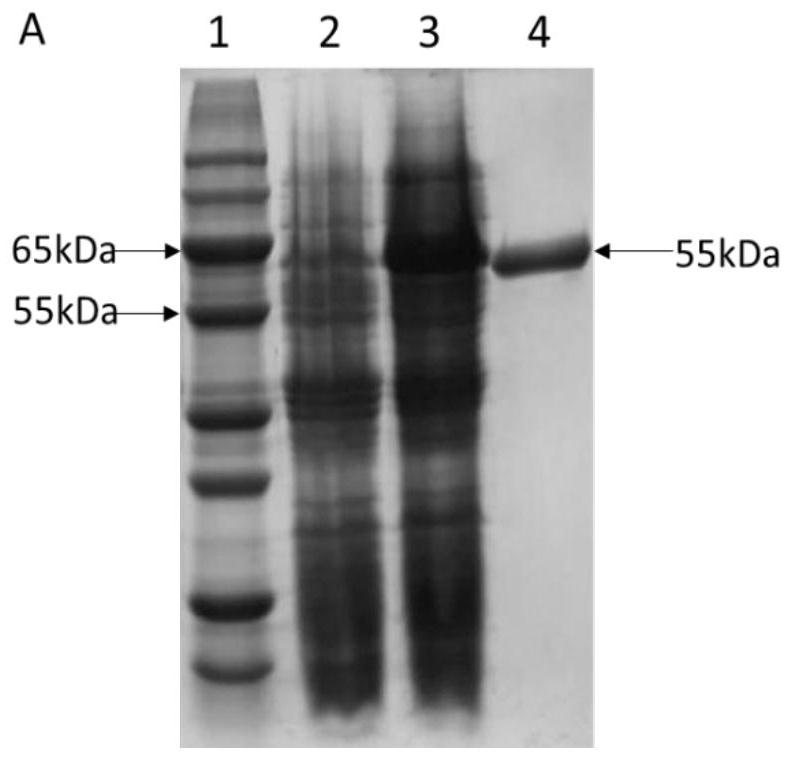
[0041] Recombinant E. coli BL21 / pANY1- araA Inoculate in LB liquid medium (yeast powder 10g / L, tryptone 20g / L, sodium chloride 20g / L), shake culture at 37°C and 200rpm until the OD is about 0.4, add IPTG (isopropyl alcohol) with a final concentration of 1mM β-D-thiogalactoside) induction, 25 ℃, 150 rpm low-speed overnight induction of LPAI, the expression of DTE-CM was detected by SDS-PAGE, and the recombinant bacteria induced without IPTG were used as the blank control, and then Will express successful LPAI utilizing Ni 2+ -NTI column purification, such as figure 2 As shown, lane 1 is the marker, lane 2 is the sample without adding inducer, lane 3 is the sample after the induction table, and lane 4 is the sample treated with Ni 2+ -The sample after NTI column purification, a single pure protein band with a size of 55kDa, the pure enzyme sample is preserved, named LPAI, and...
Embodiment 3
[0045] In order to investigate the enzymatic properties of the recombinant L-arabinose isomerase LPAI, the enzymatic activity of the recombinant L-arabinose isomerase LPAI under different metal ions and concentrations, different temperatures and pH environments were studied respectively.
[0046] (1) Effect of temperature on LPAI enzyme activity:
[0047] Set up multiple groups of experiments, add LPAI to the D-galactose substrate solution, and carry out the reaction for 1 hour at 30, 35, 40, 45, 50, 55, 60 and 65°C respectively, and measure the enzyme activity at different temperatures , to obtain the optimum temperature of the enzyme. Such as image 3 As shown, as the temperature increased, the enzyme activity became higher and higher, and when the temperature was 45°C, the enzyme activity reached the highest. Then the temperature increased again, and the enzyme activity gradually decreased.
[0048] After incubating the LPAI in the above test group for two hours, its res...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com