Preparation and application of carboxylic acid modified cyclodextrin chiral chromatographic stationary phase material
A technology of chiral chromatography and cyclodextrin, which is applied in the field of high performance liquid chromatography chiral separation and composite materials, can solve the problems of chiral separation limitations, limited racemate types, and complicated preparation methods, so as to avoid complicated preparation , avoid the use of toxic reagents, the effect of high load
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
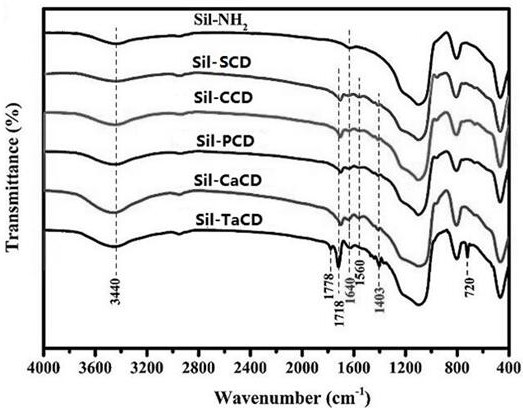
[0041] weigh β -CD (0.4~0.6g) and succinic anhydride (0.5~0.6g), dissolved in the deep eutectic solvent formed by choline chloride and ethylene glycol (choline chloride 40~50g, ethylene glycol 50~ 60 mL); heated to 80~85°C and kept stirring for 30 minutes, added aminopropyl silica gel (2.5~3.0g), carbodiimide (0.05~0.1g) and L-phenylalanine (0.5~0.6g ), continue to react for 10~12h; filter after the reaction, and wash the solid product with 50~60mL acetone and 100~120 mL methanol respectively for 2~3 times; then dry it in a vacuum oven at 55~60℃ for 8~10 hours to obtain Pale yellow powdery solid, which is modified by carboxylic acid β - Cyclodextrin chiral material, labeled Sil-SCD.
[0042] Sil-SCD was packed into a column as the stationary phase. In reverse phase mode, using acetonitrile / water as mobile phase, flavanone, α-phenylethanol, 6-methoxyflavanone, benzoin, benzoin methyl ether, benzoin ethyl ether, 1-phenyl-1-propane Alcohol, 1-(4-methylphenyl)ethanol, and tria...
Embodiment 2
[0047] Will β -CD (0.4~0.6 g) and butenedioic anhydride (0.5~0.6g) were dissolved in the deep eutectic solvent formed by choline chloride and ethylene glycol (choline chloride 40~50g, ethylene glycol 50~ 60mL); warm up to 80~85℃ and keep stirring for 30 minutes; add aminopropyl silica gel (2.5~3.0 g), carbodiimide (0.05~0.1g) and L-phenylalanine (0.5~0.6g) , continue to react for 10~12h; filter after the reaction, wash the solid product with 50~60mL acetone and 100~120 mL methanol for 2~3 times, and then dry in a vacuum oven at 60°C for 8~10 hours to obtain a light yellow powder Shaped solid, that is, carboxylic acid modified β-cyclodextrin chiral material, marked as Sil-CCD.
[0048] Packed into a column as a stationary phase. Determination in the reverse phase mode, using acetonitrile / water as mobile phase, with flavanone, α-phenylethanol, 6-methoxyflavanone, benzoin, benzoin methyl ether, benzoin ethyl ether, 1-phenyl-1 -Propanol, 1-(4-methylphenyl)ethanol, and triadimen...
Embodiment 3
[0051] Will β -CD (0.4~0.6 g) and phthalic anhydride (0.5~0.6 g) were dissolved in the deep eutectic solvent formed by choline chloride and ethylene glycol (choline chloride 40~50 g, ethylene glycol 50 ~60mL); warm up to 80~85°C and keep stirring for 30 minutes; add aminopropyl silica gel (2.5~3.0 g), carbodiimide (0.05~0.1g) and L-phenylalanine (0.5~0.6g ), continue to react for 10~12h, filter after the reaction, wash the solid product with 50~60mL acetone and 100~120 mL methanol for 2~3 times, and then dry it in a vacuum oven at 60°C for 8~10 hours to obtain light yellow Powdered solid, that is, carboxylic acid modification β - Cyclodextrin chiral material, labeled as Sil-PCD.
[0052] Packed into a column as a stationary phase. In reverse phase mode, using acetonitrile / water as mobile phase, flavanone, α-phenylethanol, 6-methoxyflavanone, benzoin, benzoin methyl ether, benzoin ethyl ether, 1-phenyl-1-propane Alcohol, 1-(4-methylphenyl)ethanol, and triadimenol were used ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com